New anode material could lead to safer fast-charging batteries
Published Date
Article Content
Scientists at UC San Diego have discovered a new anode material that enables lithium-ion batteries to be safely recharged within minutes for thousands of cycles. Known as a disordered rocksalt, the new anode is made up of earth-abundant lithium, vanadium and oxygen atoms arranged in a similar way as ordinary kitchen table salt, but randomly. It is promising for commercial applications where both high energy density and high power are desired, such as electric cars, vacuum cleaners or drills.
The study, jointly led by nanoengineers in the labs of Professors Ping Liu and Shyue Ping Ong, was published in Nature on September 2.
Currently, two materials are used as anodes in most commercially available lithium-ion batteries that power items like cell phones, laptops and electric vehicles. The most common, a graphite anode, is extremely energy dense—a lithium ion battery with a graphite anode can power a car for hundreds of miles without needing to be recharged. However, recharging a graphite anode too quickly can result in fire and explosions due to a process called lithium metal plating. A safer alternative, the lithium titanate anode, can be recharged rapidly but results in a significant decrease in energy density, which means the battery needs to be recharged more frequently.
This new disordered rocksalt anode-- Li3V2O5 -- sits in an important middle ground: it is safer to use than graphite, yet offers a battery with at least 71% more energy than lithium titanate.
“The capacity and energy will be a little bit lower than graphite, but it’s faster, safer and has a longer life. It has a much lower voltage and therefore much improved energy density over current commercialized fast charging lithium-titanate anodes,” said Haodong Liu, a postdoctoral scholar in Professor Ping Liu’s lab and first author of the paper. “So with this material we can make fast-charging, safe batteries with a long life, without sacrificing too much energy density.”
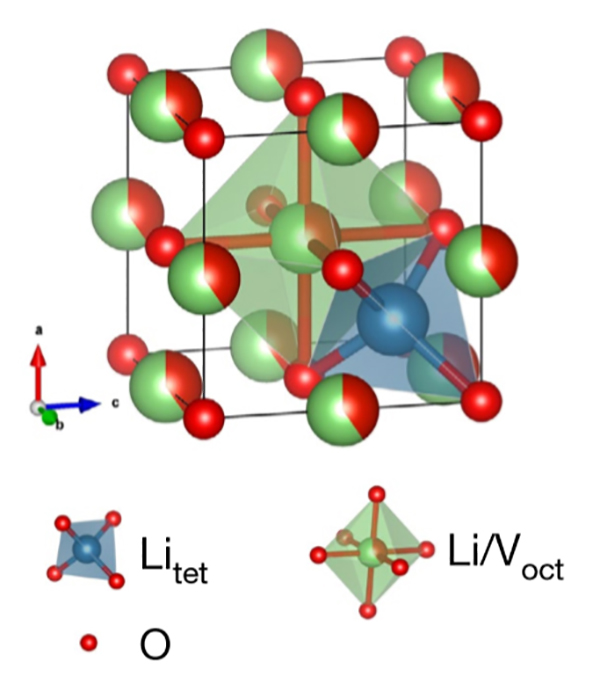
The crystal structure of disordered rocksalt -Li3V2O5. The red balls represent O, the blue tetrahedron represents Li in tetrahedral sites, and the green octahedron represents the Li/V shared octahedral sites
The researchers formed a company called Tyfast in order to commercialize this discovery. The startup’s first markets will be electric buses and power tools, since the characteristics of the Li3V2O5 disordered rocksalt make it ideal for use in devices where recharging can be easily scheduled.
Researchers in Professor Liu’s lab plan to continue developing this lithium-vanadium oxide anode material, while also optimizing other battery components to develop a commercially viable full cell.
“For a long time, the battery community has been looking for an anode material operating at a potential just above graphite to enable safe, fast charging lithium-ion batteries. This material fills an important knowledge and application gap,” said Ping Liu. “We are excited for its commercial potential since the material can be a drop-in solution for today’s lithium-ion battery manufacturing process.”
Why try this material?
Researchers first experimented with disordered rocksalt as a battery cathode six years ago. Since then, much work has been done to turn the material into an efficient cathode. Haodong Liu said the UC San Diego team decided to test the material as an anode based on a hunch.
“When people use it as a cathode they have to discharge the material to 1.5 volts,” he said. “But when we looked at the structure of the cathode material at 1.5 volts, we thought this material has a special structure that may be able to host more lithium ions—that means it can go to even lower voltage to work as an anode.”
In the study, the team found that their disordered rocksalt anode could reversibly cycle two lithium ions at an average voltage of 0.6 V—higher than the 0.1 V of graphite, eliminating lithium metal plating at a high charge rate which makes the battery safer, but lower than the 1.5 V at which lithium-titanate intercalates lithium, and therefore storing much more energy.
The researchers showed that the Li3V2O5 anode can be cycled for over 6,000 cycles with negligible capacity decay, and can charge and discharge energy rapidly, delivering over 40 percent of its capacity in 20 seconds. The low voltage and high rate of energy transfer are due to a unique redistributive lithium intercalation mechanism with low energy barriers.
Postdoctoral scholar Zhuoying Zhu, from Professor Shyue Ping Ong’s Materials Virtual Lab, performed theoretical calculations to understand why the disordered rocksalt Li3V2O5 anode works as well as it does.
“We discovered that Li3V2O5 operates via a charging mechanism that is different from other electrode materials. The lithium ions rearrange themselves in a way that results in both low voltage as well as fast lithium diffusion,” said Zhuoying Zhu.
“We believe there are other electrode materials waiting to be discovered that operate on a similar mechanism,” added Ong.
The corresponding and first authors on a Zoom call. From top left clockwise: Professor Ping Liu, Professor Shyue Ping Ong, Haodong Liu, Jun Lu, Professor Huolin Xin, and Zhuoying Zhu.
The experimental studies at UC San Diego were funded by awards from the UC San Diego startup fund to Ping Liu, while the theoretical studies were funded by the Department of Energy and the National Science Foundation’s Data Infrastructure Building Blocks (DIBBS) Local Spectroscopy Data Infrastructure program, and used resources at the San Diego Supercomputer Center provided under the Extreme Science and Engineering Discovery Environment (XSEDE).
The team also collaborated with researchers at Oak Ridge National Lab, who used neutron diffraction to determine the atomic structure of the Li3V2O5 material. Researchers at UC Irvine and Brookhaven National Lab led by Professor Huolin Xin performed high resolution microscopic studies to resolve the structural changes after lithium insertion. Finally, the teams at Argonne National Lab led by Jun Lu, and Lawrence Berkeley National Lab, conducted X-ray diffraction and X-ray absorption studies to reveal the crystal structural change and charge compensation mechanisms of the material during (de)lithiation. This study used national lab facilities including the beamline VULCAN (Spallation Neutron Source at Oak Ridge National Lab), beamline 17-BM (Advanced Photon Source at Argonne National Lab), beamline 5.3.1 (Advanced Light Source at Lawrence Berkeley National Lab).
Paper Title: “A disordered rock salt anode for fast-charging lithium-ion batteries.” Co-authors include Haodong Liu, Zhuoying Zhu, Qizhang Yan, Sicen Yu, Yiming Chen, Yejing Li, Xing Xing, Yoonjung Choi, Shyue Ping Ong and Ping Liu, UC San Diego; Xin He, Jun Feng, Robert Kostecki, Lawrence Berkeley National Laboratory; Yan Chen, Ke An, Oak Ridge National Laboratory; Rui Zhang, Huolin L. Xin, University of California; Lu Ma, Ruoqian Lin, Brookhaven National Laboratory; Tongchao Liu, Matthew Li, Khalil Amine, Tianpin Wu, Jun Lu, Argonne National Laboratory; Lucy Gao, Del Norte High School; Helen Sung-yun Cho, Canyon Crest Academy.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.