Drug Targets Hard-to-Reach Leukemia Stem Cells Responsible for Relapses
By:
Published Date
Article Content
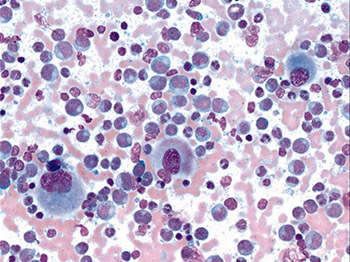
Chronic myeloid leukemia leads to production of many abnormal white blood cells, which do not fight infection as well as normal white blood cells. As these abnormal cells accumulate in blood and bone marrow, they crowd out healthy white blood cells, red blood cells and platelets, impairing normal functions.
Researchers at the University of California, San Diego School of Medicine have discovered that hard-to-reach, drug-resistant leukemia stem cells (LSCs) that overexpress multiple pro-survival protein forms are sensitive – and thus vulnerable – to a novel cancer stem cell-targeting drug currently under development.
The findings, published in the January 17 online issue of Cell Stem Cell, open the possibility that diseases like chronic myeloid leukemia (CML) and some solid tumor cancers might – in combination with other therapies – be more effectively treated with this drug, and with a lower chance of relapse.
Led by principal investigator Catriona H. M. Jamieson, MD, PhD, associate professor of medicine and director of stem cell research at UC San Diego Moores Cancer Center, the researchers found that a compound called sabutoclax appears to selectively target LSCs that express particular protein isoforms through alternatively splicing, a fundamental process in which a gene is able to code for multiple proteins.
An emerging class of drugs called tyrosine kinase inhibitors (TKI) – such as imitinib (Gleevec), gifitinib (Iressa) and sunitinib (Sutent) – has become a popular anti-cancer treatment. However, current TKIs are not 100 percent effective. In cases of CML, for example, some LSCs tucked protectively within bone marrow elude destruction, develop resistance to therapy, self-renew and eventually cause the leukemia to dramatically return.
Jamieson and colleagues found that alternative splicing of BCL2 genes, which code for proteins involved in apoptosis or programmed cell death, specifically promoted malignant transformation of dormant white blood cell precursors into “blast crisis” LSCs. The blast crisis is the final phase of CML when overabundant, abnormal white blood cells crowd out healthy cells, causing serious dysfunction.
Of clinical importance, they noted that sabutoclax, which suppresses all BCL2 anti-apoptotic proteins, renders these marrow-dwelling blast crisis LSCs sensitive – and more susceptible – to TKI-based therapeutics at doses that do not harm normal progenitor cells.
“Our findings show that pan-BCL2 inhibition will be critical for the eradication of cancer stem cells in CML and that there is an essential link between cancer stem cell dormancy, pro-survival BCL2 isoform expression and therapeutic resistance,” Jamieson said. “By using a novel pan-BCL2 inhibitor, we may be able to prevent therapeutic resistance by sensitizing malignant stem cell clones to TKIs.”
The findings may have implications for treating solid tumor cancers, such as colon, prostate, breast, and brain cancers, noted Daniel J. Goff, the study’s first author. “With many of these tumor types being shown to harbor cancer stem cells, it raises the question of whether BCL2 family expression as well as isoform-switching may be crucial for the maintenance of cancer stem cells in these diseases as well,” he said. “If so, they may also be candidates for treatment with a BCL2 inhibitor like sabutoclax.”
Co-authors are Angela Court Recart, Anil Sadarangani, Heather Leu, Janine Low-Marchelli, Wenxue Ma, Alice Y. Shih, Ifat Geron, Minya Pu, Lei Bao, Ryan Chuang, Larisa Balaian, Peggy Wentworth, Kristen M. Smith, Christina A.M. Jamieson, Sheldon R. Rorris and Karen Messer, UCSD Department of Medicine and UCSD Moores Cancer Center; Hye-Jung Chun and Marco Marra, Michael Smith Genome Sciences Center, Vancouver, BC, Canada; Christian L. Barrett and Kelly A. Frazer, UCSD Department of Pediatrics; Maryla Krajewska, Jun Wei, Dayong Zhai, Maurizio Pellecchia and John C. Reed, Sanford-Burnham Medical Research Institute; Jason Gotlib, Stanford Medical Center; Mark Minden, Princess Margaret Hospital, Toronto, Canada; Giovanni Martinelli, Institute of Hematology and Medical Oncology, University of Bologna, Italy; Jessica Rusert and Lawrence S.B. Goldstein, UCSD Department of Cellular and Molecular Medicine and Howard Hughes Medical Institute; Kim-Hien Dao, Oregon Health and Science University, Portland; Kamran Shazand and Thomas J. Hudson, Ontario Institute for Cancer Research, Toronto, Canada.
Funding for this research was provided by a California Institute for Regenerative Medicine (CIRM) early Translational II grant (TR2-1789), a CIRM HALT leukemia disease team grant (DR1-01430), the UCSD CIRM Training Grant (TG2-01154), the Ratner Family Foundation, the National Cancer Institute (CA-55164), the National Institutes of Health (CA-149668), the Ontario Institute for Cancer Research, Genome Canada, Ontario Genomics Institute and the Canadian Institute of Health Research.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.