A New Strategy to Transform Liver Cancer Immunotherapy
For most liver cancer patients, immunotherapy does not produce effective results but new data suggests a revamped strategy can make unresponsive liver tumors highly responsive
Published Date
Article Content
In recent years, tumor immunotherapy has emerged as a highly promising and much-touted oncological approach. It is based on using humanized antibodies called immune checkpoint inhibitors (ICIs) to block the cellular pathways that inhibit the activity of T lymphocytes, a type of immune system cells that help protect the body from infection and may help fight cancer.
The best-known antibodies are those raised against CTLA-4, PD-L1 and PD-1.
Yet despite encouraging outcomes in using immunotherapy to treat some types of malignancy, most cancer patients respond poorly or not at all to treatment using ICIs, most notably patients with liver cancer.
As a result, researchers have sought ways to improve the efficacy of immunotherapy, specifically by combining more than one treatment. Several “combinatorial therapies” are currently in clinical trials, but no preclinical data or clear rationale has yet appeared to lend credence to the combined treatment approach.
In a new study, published online December 3, 2021, in the journal Hepatology, researchers at University of California San Diego, led by senior author Gen-Sheng Feng, PhD, professor of pathology in the School of Medicine and of molecular biology in the Division of Biological Sciences, demonstrate a proof of principle that liver cancer can be rendered highly responsive to an immune checkpoint inhibitor known as anti-PD-L1 antibody by using a synthetic dsRNA molecule dubbed polyIC in tandem to boost the liver’s innate immunity.
“There are two issues that we must keep in mind in developing liver cancer immunotherapy” said Feng. “In general, the tumor microenvironment is typically featured by immune suppression, otherwise tumors won’t grow out. Another level of complexity is that the liver is a uniquely immune-tolerant organ constantly exposed to foreign substances taken from foods. A successful immunotherapy must rely on overcoming hepatic immune-tolerance and disrupting the immune evasion mechanism in the tumor microenvironment.”
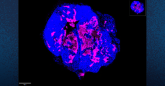
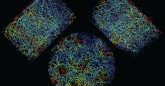
To address these issues, Feng and colleagues generated two mouse tumor models, one with tumors growing under the skin and another with tumors growing within the liver. Both types of tumor were derived from the same colorectal cancer cell line, which allowed the researchers to specifically investigate the role of the different tumor microenvironment.
They compared the responses of subcutaneous and liver tumors to the same treatment using the anti-PD-L1-antibody, polyIC molecule or the combination of both. They found that polyIC or anti-PD-L1 monotherapy effectively suppressed subcutaneous tumor growth, consistent with previous public reports. No single monotherapy produced significant therapeutic effect on tumors growing within the mouse liver.
But Feng and colleagues found that the combination of the two reagents showed remarkably synergistic effects in liver tumor inhibition, even better than in subcutaneous tumors. Their analyses suggest this was largely due to efficiently boosting the cytotoxic CD8 T cell subpopulation in the liver, simultaneously activating innate immune cells by polyIC and blocking the inhibitory pathway in T lymphocytes with the anti-PD-L1 antibody.
“The detailed molecular and cellular mechanisms underlying the synergistic effect of this combination are yet to be deciphered,” said Feng. “The reason we are excited is that the data suggest a possibility that liver cancer can be turned highly responsive to immunotherapy, as long as one can find a way to overcome the immune-suppressive liver environment. There is a promising future that liver cancer patients can benefit from immunotherapy.”
The study, noted the authors, also raised a caution regarding immunotherapy research of subcutaneous tumor model. “This model was used in the pioneering work for cancer immunotherapy,” said Feng, “and it remains the most frequently used animal tumor model in the cancer immunology. However, our studies disclosed a limited value of the subcutaneous tumor model in dissecting the unique liver tumor microenvironment.”
Co-authors include: Bing Xin, Meixiang Yang, Panyisha Wu, Li Du, Hester Deng, Enfu Hui, all at UC San Diego.
Funding for this research came, in part, from the National Cancer Institute (grants R01CA236074, R01CA239629, R37CA239072), the National Institutes of Aging (P01AG073084) and the Krueger v. Wyeth research award.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.