This Injected Protein-like Polymer Helps Tissues Heal After a Heart Attack
Story by:
Published Date
Article Content
Researchers have developed a new therapy that can be injected intravenously right after a heart attack to promote healing and prevent heart failure.
The therapy both prompts the immune system to encourage tissue repair and promotes survival of heart muscle cells after a heart attack. Researchers tested the therapy in rats and showed that it is effective up to five weeks after injection.
The research team, led by bioengineers at the University of California San Diego and chemists at Northwestern University, published their findings in the April 25 issue of the journal Advanced Materials.
“Preventing heart failure after a heart attack is still a major unmet clinical need,” said Karen Christman, one of the study’s corresponding authors and a professor in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering. “The goal of this therapy is to intervene very soon after someone suffers a heart attack to keep them from ultimately going into heart failure.”
The therapy could have broader applications, said Nathan Gianneschi, the paper’s other corresponding author and a professor in the Department of Chemistry at Northwestern.
“This therapeutic platform has tremendous potential for several diseases, including everything from macular degeneration to multiple sclerosis and kidney disease,” Gianneschi said.
The platform aims to block the interaction of two key proteins that intervene in the body’s response to stress and inflammation. When the protein Nrf2 is activated, cells resist the degradation brought on by inflammation. But KEAP1 binds with Nrf2 to degrade it in turn. After a heart attack, this process of degradation has to be stopped so that tissues can health better.
The protein-like polymer, or PLP, platform is made from a polymer that mimics Nrf2. Once injected intravenously, it finds KEAP1 and binds to it, preventing it from binding to the actual Nrf2 protein and degrading it.
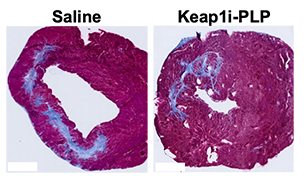
Researchers injected rat models after a heart attack with either the PLP platform or a saline solution. The team was blinded to which animals received the polymer or saline. After five weeks, the rodents underwent MRIs while sedated. The animals injected with the polymer showed better cardiac function and significantly more healing in their heart muscle tissue. Other tests also showed that genes that promote healing of tissues were expressed more.
Researchers describe the study as a proof of concept. Before moving on to tests in larger mammals, they want to optimize the design and dosage, and conduct further analysis.
“Proteins are the molecular machines that drive all essential cellular function, and dysregulated intracellular protein-protein interactions are the cause of many human diseases,” Gianneschi said. “Existing drug modalities are either unable to penetrate cells or cannot effectively engage these large disease target domains. We are looking at these challenges through a new lens.”
The therapy method was developed by Gianneschi, while he was a faculty member at UC San Diego, where he is now an adjunct faculty. He continued working on the technology at Northwestern.
This work was made possible by research grants from the National Institutes of Health National Heart, Lung, and Blood Institute (NHLBI)(2R01HL139001, R00 CA248715).
Gianneschi is a co-founder of Grove Biopharma, which licensed intellectual property related to the study. Gianneschi and Christman are co-inventors on that same intellectual property. Gianneschi also serves on the Scientific Advisory Board for Grove Biopharma.

Protein-like Polymers Targeting Keap1/Nrf2 as Therapeutics for Myocardial Infarction
J. M. Mesfin, Q. P. Lyons, M. B. Nguyen, J. D. Hunter, E. G. Wong, K. Reimold, S. N. Paleti, E. Gardner, C. G. Luo, Karen Christman
Shu Chien-Gene Lay Department of Bioengineering, Sanford Consortium for Regenerative Medicine, Sanford Stem Cell Institute, University of California San Diego, La Jolla, California 92037, United States
A. Chen
Program in Materials Science and Engineering, Sanford Consortium for Regenerative Medicine, University of California San Diego, La Jolla, California 92037, United States
K. P. Carrow
Medical Scientist Training Program, Department of Biomedical Engineering, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611, United States
M. P. Hopps, J. J. Holm, M. P. Thompson
Department of Chemistry, International Institute for Nanotechnology, Simpson-Querrey Institute, Chemistry of Life Processes Institute, Northwestern University, Evanston, Illinois 60208, United States
A. Magassa, X. Zhang
Department of Chemistry, Department of Materials Science & Engineering, Department of Pharmacology, International Institute for Nanotechnology, Simpson-Querrey Institute, Chemistry of Life Processes Institute, Northwestern University, Evanston, Illinois 60208, United States
N. C. Gianneschi
Department of Biomedical Engineering, Department of Chemistry, Department of Materials Science & Engineering, Department of Pharmacology, International Institute for Nanotechnology, Chemistry of Life Processes Institute, Northwestern University, Evanston, Illinois 60208, United States
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



