Study Uncovers Metabolic Cause for Rare Eye Disease
Published Date
By:
- Liezel Labios
Share This:
Article Content
An international team of researchers has discovered a cause for a rare eye disease affecting the macula that leads to loss of central vision, called macular telangiectasia type 2 (MacTel). Using genetic and metabolic data from patients with MacTel, researchers found that the disease is driven by reduced levels of the amino acid serine and accumulation of toxic lipids called deoxysphingolipids.
The team, co-led by the Lowy Medical Research Institute, the University of California San Diego, the Scripps Research Institute and the University of Utah, published their findings Sept. 11 in the New England Journal of Medicine.
“Through an interdisciplinary collaborative effort, we show how a combination of genetics, metabolism and biochemistry drives a disease such as MacTel,” said co-senior author Christian Metallo, a UC San Diego professor of bioengineering whose lab explores how metabolism contributes to disease. “In this case, there was no single gene driving the disease, which made it an extraordinarily complex and cool puzzle to help piece together.”
MacTel is a debilitating eye disease that affects about two million people worldwide, most of whom are at least 50 years old. It is a disease of the macula, the central area of the retina, and causes those affected to experience a gradual deterioration of central vision, interfering with tasks such as reading and driving. There are currently no treatments available for patients.
In previous work, researchers discovered genetic mutations related to serine metabolism in some MacTel patients, who generally had lower serine in blood compared to unaffected patients. However, it was still unclear how low blood serine levels drive the disease.
To answer this question, the researchers took a detailed look at serine metabolism. They found that low serine levels in patients with MacTel lead to the production of toxic fatty molecules called deoxysphingolipids that damage the eye.
This all has to do with a particular biochemical reaction involved in serine metabolism. The enzyme serine palmitoyl-transferase typically uses serine to make fatty molecules called sphingolipids, which are essential for cell function. But if serine levels are low, the enzyme can act “promiscuously” and use a different amino acid such as alanine, which results in the production of toxic deoxysphingolipids.
“A key contribution of this study is the strong correlation between amino acids and lipid diversity in the blood. This explains how changes in serine (or alanine) metabolism could impact production of toxic lipids that cause disease,” Metallo said.
Another clue that led to this discovery came from a family in Lowy Medical Research Institute’s patient network. Two members of this family are affected by both MacTel and another rare disease caused by deoxysphingolipid production, called hereditary sensory and autonomic neuropathy type 1 (HSAN1). To see if there was a connection, LMRI researchers conducted comprehensive eye exams on a group of 13 patients with HSAN1. They found the majority also had MacTel.
“We had a hunch that deoxysphingolipids might be important for MacTel, but finding out about the HSAN1 patients really encouraged us to dig deeper and establish the link between these two diseases,” said co-first author Martina Wallace, a postdoctoral researcher in Metallo’s lab.
In mice, the researchers also found that low serine levels are correlated with high deoxysphingolipid levels, which result in reduced peripheral and retinal nerve function.
“Strikingly, when we artificially lowered serine levels in mice by feeding them engineered diets, we could induce retinal defects and peripheral neuropathy in the animals reminiscent of the phenotypes observed in these patients,” Metallo said.
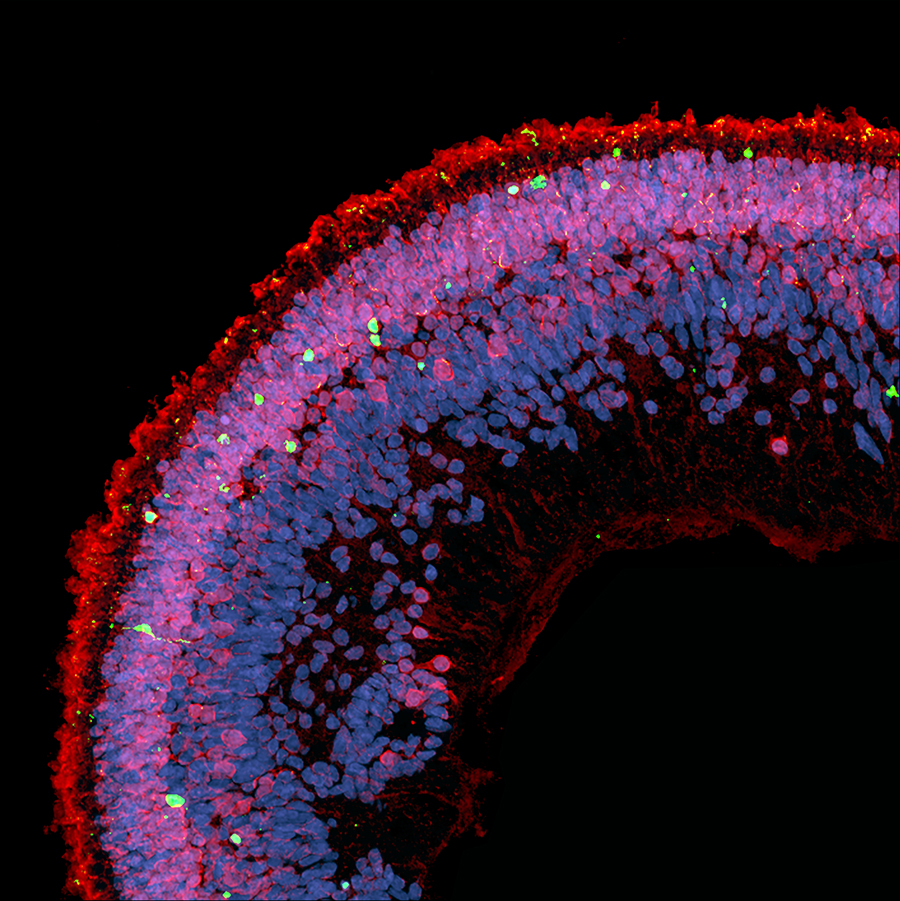
Cell death (green) is observed in a cross-section of a retinal organoid exposed to deoxysphingolipids. Photoreceptors are red, cell nuclei are blue. Image credit: Kevin Eade/LMRI
To further examine how deoxysphingolipids affect human retinal tissues, the researchers created retinal organoids from human induced pluripotent stem cells and then exposed them to deoxysphingolipids. They found that high levels caused photoreceptor cell death in the retinal organoids.
They also found that treating the retinal organoids with a drug for high cholesterol, called fenofibrate, protected against photoreceptor cell death by promoting the breakdown of deoxysphingolipids.
The next steps in the research are to examine how widespread deoxysphingolipids are in macular disease and to characterize the pathways that lead to elevated levels of deoxysphingolipids in MacTel. Ongoing studies in Metallo’s lab are exploring how serine metabolism influences cancer growth, as well as how diet and genetics impact lipid metabolism to drive neuropathy.
Paper title: “Serine and Lipid Metabolism in Macular Disease and Peripheral Neuropathy.” Co-authors include Michal K. Handzlik, Michelle Baldini, and Mehmet G. Badur of UC San Diego; Marin L. Gantner*, Kevin Eade*, Regis Fallon, Jennifer Trombley, Sarah Harkins-Perry, Sarah Giles, Lea Scheppke, Michael I. Dorrell and Martin Friedlander of Lowy Medical Research Institute; Lydia Sauer, Barbara J. Hart and Paul Bernstein of University of Utah; Catherine Egan of Moorfields Eye Hospital; Yoichiro Ideguchi and Maki Kitano of The Scripps Research Institute; Rando Allikmets, Carolyn Cai, and Takayumi Nagasaki of Columbia University; Roberto Bonelli and Melanie Bahlo of Walter and Eliza Hall Institute of Medical Research; Mali Okada of Royal Victorian Eye and Ear Hospital; Tjebo FC. Heeren, Sasha M. Woods and Marcus Fruttiger of University College London Institute of Ophthalmology; Mark Gillies of University of Sydney; Robyn Guymer of University of Melbourne Centre for Eye Research; and Florian Eichler of Massachusetts General Hospital.
*These authors contributed equally to this work
This work was supported by the Lowy Medical Research Institute, the National Science Foundation (CAREER Award #1454425), the National Institutes of Health (grant NCI R01CA188652), The National Health and Medical Research Council of Australia, National Eye Institute, and the University of Melbourne Research Scholarship Program.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



