Stealth Genetic Switch in Mosquitoes Halts Malaria Spread
Novel system uses CRISPR to replace one molecule and block parasites that cause malaria infection
Published Date
Story by:
Topics covered:
Share This:
Article Content
Mosquitoes kill more people each year than any other animal. In 2023, the blood-sucking insects infected a reported 263 million people with malaria, leading to nearly 600,000 deaths, 80% of which were children.
Recent efforts to block the transmission of malaria have been stalled because mosquitoes have adapted resistance to insecticides and the parasites within mosquitoes that cause malaria have become resistant to drugs. These setbacks have been amplified by the COVID-19 pandemic, which impeded ongoing anti-malarial efforts.
Now, researchers at the University of California San Diego, Johns Hopkins University, UC Berkeley and the University of São Paulo have developed a new method that genetically blocks mosquitoes from transmitting malaria. The study was published July 23, 2025 in the journal Nature.
Biologists Zhiqian Li and Ethan Bier from UC San Diego, and Yuemei Dong and George Dimopoulos from Johns Hopkins University, created a CRISPR-based gene-editing system that changes a single molecule within mosquitoes, a minuscule but effective change that stops the malaria-parasite transmission process. Genetically altered mosquitoes are still able to bite those with malaria and acquire parasites from their blood, but the parasites can no longer be spread to other people. The new system is designed to genetically spread the malaria resistance trait until entire populations of the insects no longer transfer the disease-causing parasites.
“Replacing a single amino acid in mosquitoes with another naturally occurring variant that prevents them from being infected with malarial parasites — and spreading that beneficial trait throughout a mosquito population — is a game-changer,” said Bier, a professor in the UC San Diego Department of Cell and Developmental Biology (School of Biological Sciences). “It’s hard to believe that this one tiny change has such a dramatic effect.”
The newly developed system uses CRISPR-Cas9 “scissors” and a guide RNA to make a genetic cut at a precise location within the mosquito’s genome. It then replaces the unwanted amino acid that transmits malaria with the beneficial version that does not.
The system targets a gene that produces a protein known as “FREP1” that helps mosquitoes develop and feed on blood when they bite. The new system switches an amino acid in FREP1 known as L224 with a genetic alternate, or allele, called Q224. Disease-causing parasites use L224 to swim to the insect’s salivary glands, where they are positioned to infect a person or animal.
Dimopoulos, a professor in the Department of Molecular Microbiology and Immunology and the Johns Hopkins Malaria Research Institute (Bloomberg School of Public Health), and his lab tested strains of Anopheles stephensi mosquitoes, the main vector of malaria transmission in Asia. They found that the L224-to-Q224 switch could effectively block two different types of malarial parasites from reaching the salivary glands, thereby preventing infection.

Mosquitoes that readily transmit malarial parasites carry the FREP1 amino acid known as L224 (red dots inside mosquitoes and marked with “L”). The newly developed system uses an allelic gene drive system (scissors) to convert mosquitoes into a population that now carries the malaria-suppressing Q224 allele (highlighted in green and marked with “Q”). Credit: Audrey Yeun, Bier Lab, UC San Diego

“The beauty of this approach lies in leveraging a naturally occurring mosquito gene allele,” said Dimopoulos. “With a single, precise tweak, we’ve turned it into a powerful shield that blocks multiple malaria parasite species and likely across diverse mosquito species and populations, paving the way for adaptable, real-world strategies to control this disease.”
In a range of follow-on tests, the researchers found that although the genetic switch disrupted the parasite’s infection capabilities, the mosquitoes’ normal growth and reproduction remained unchanged. Mosquitoes carrying the newly inserted variant Q224 exhibited similar fitness to those with the original L224 amino acid, a key achievement since the FREP1 protein plays an important role in the biology of the mosquito, which is separate from its role in being exploited by malarial parasites.
Similar to a gene-drive, the researchers created a technique for mosquito offspring to genetically inherit the Q224 allele and spread it throughout their populations, halting the transmission of malaria parasites. This new “allelic-drive” follows a comparable system recently engineered in the Bier Lab that genetically reverses insecticide resistance in crop pests.
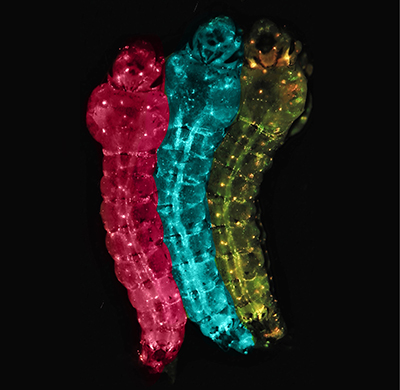
Genetically modified mosquito larvae express fluorescent markers of the FREP1 gene — RFP (pink), GFP (blue) or both (yellow) — to indicate whether they spread or block infection from malarial parasites. Credit: Zhiqian Li, Bier Lab, UC San Diego
“In that prior study, we created a self-eliminating drive that converts a population of fruit flies from being resistant to insecticides back to its native insecticide-susceptible state. Then that genetic cassette just disappears, leaving only a re-wilded insect population,” said Bier. “A similar phantom drive system could convert mosquito populations to carrying the parasite-resistant FREP1Q variant.”
While the researchers demonstrated the effectiveness of the L224-to-Q224 switch, they don’t yet fully grasp why this change works so efficiently. Ongoing research into how the Q224 amino acid blocks the parasite’s infection transit route is underway.
“This breakthrough is the result of seamless teamwork and innovation across institutions,” said Dimopoulos. “Together, we’ve harnessed nature’s own genetic tools to turn mosquitoes into allies against malaria.”
The authors of the study were: Zhiqian Li, Yuemei Dong, Lang You, Rodrigo M. Corder, Jemariz Arzobal, Audrey Yeun, Lei Yang, John M. Marshall, George Dimopoulos and Ethan Bier.
Funding for the research was provided by: the Bill & Melinda Gates Foundation (INV-036579, INV-043645, INV-017683 and INV-078535); the National Institutes of Health (grants R01GM117321, R01GM144608, R01AI162911, R01AI170692, R01AI158615 and R01AI143698); Howard Hughes Medical Institute (grant 90101319); Bloomberg Philanthropies; and Open Philanthropy (grant GV673605686).
Competing interest disclosure: Bier has equity interest in two companies he co-founded: Agragene Inc. and Synbal Inc., which may potentially benefit from the research results.
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.





