UC San Diego Researchers Show Molecules Change Their Behaviors Under a Polariton Leader
Story by:
Published Date
Article Content
In recent years, manipulating chemistry using hybrid light-matter states called polaritons has generated much research as it combines the speed and efficiency of light with the reactivity and strong interactions of matter. Vibrational polaritons are formed when a specific vibrational motion of the molecule and photon creates a “spring” that allows them to quickly exchange energy. This is called vibrational strong coupling (VSC).
Although much effort has been devoted to finding a sound explanation for VSC-modified chemistry and whether vibrational polaritons can alter molecular dynamics, consensus between theory and experiment has been lacking.
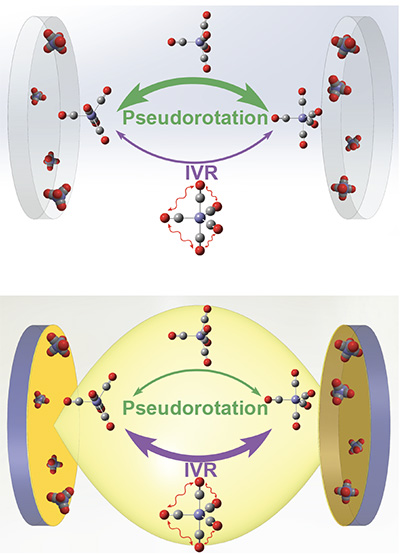
The question University of California San Diego Professors of Chemistry Wei Xiong and Joel Yuen Zhou sought to answer was whether polariton modes and dark modes (the molecular byproduct of polariton creation) both modify chemical reactions. Their paper, recently published in Science, shows unambiguously that chemical reactions only occur with polaritons.
Previous experiments used complex systems that did not allow for any separation between polaritons and dark modes, making it difficult to differentiate what was occurring and impossible to understand what happened with either mode individually. To remedy this, Xiong used 2D infrared spectroscopy on a simple chemical reaction that was easier to analyze. This allowed his lab to separately excite and follow the dynamics of the polariton modes and dark modes.
“The big question in the community was whether the individual molecules inside a cavity could follow their own will,” said Xiong. “In this experiment, we showed that molecules just do the same thing over and over again on their own, until a polariton ‘leader’ brings them together.”
Xiong explains that this paper lays a foundation for continued research into controlling chemical reactions. “If a molecule performs the same reaction over and over, we’re not controlling it; we’re just observing it,” he stated. “Polaritons are a new way of controlling reactions. We need to consider ways of making molecules act together, synchronized under a photon leader, to amplify their collective power.”
“Theoretically, it’s exciting because we’re not looking at molecules one at a time; we’re looking at them as a many-body system,” said Yuen Zhou. “It’s this idea of collective chemistry and understanding what happens when all molecules decide to do the same thing. This is an exciting new avenue for chemistry at the interface with quantum many-body physics.”
This work was funded by National Science Foundation DMR-1848215 and CHE-2101988; AFSOR FA9550-21-1-0369; the Alfred P. Sloan Foundation FG-2020-12845 and the ACS PRF 60968-ND6 Grant. Additional support provided by the US Department of Energy, Office of Science, Basic Energy Sciences, CPIMS Program Early Career Research Program Award DE-SC0019188.
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



