Leukemia in Remission for First Patient to Undergo CAR T-Cell Immunotherapy in San Diego
Published Date
By:
- Heather Buschman, PhD
Share This:
Article Content
Robert Legaspi was 9 years old when he was first diagnosed with acute lymphoblastic leukemia (ALL). This year, at age 27, his leukemia returned for the fourth time. This time was different, though — on May 20, 2016, Legaspi became the first patient in San Diego to receive a new type of immunotherapy, known as CAR T-cell therapy, as part of a Phase I/II clinical trial at Moores Cancer Center at UC San Diego Health. His leukemia is now in remission again, more quickly and after a much better therapeutic experience.
“All three of my previous therapies were intense and overwhelming, especially when I was a kid, and it took two years to get back into remission each time,” Legaspi said. “You get so depleted by chemotherapy and radiation that you feel like you’re not even there anymore. And after putting that much time and energy into therapy, you’d expect a forever cure, but years later, it would come back and my life would be a nightmare again.”
Earlier this year, Legaspi had just returned home to San Diego from his wedding in the Philippines when he knew something was wrong. After several visits to the doctor, his suspicions were confirmed: the leukemia had returned for a fourth time. But it hadn’t yet reached his bone marrow. That’s when Legaspi’s oncologist, Ted Ball, MD, at Moores Cancer Center, told him about a new approach — CAR T-cells, an experimental immunotherapy that is only available at a few specific medical centers around the country.
Legaspi received CAR T-cell therapy as part of a clinical trial at Moores Cancer Center at UC San Diego Health, sponsored by Kite Pharma. Legaspi’s own T-cells, part of his immune system, were collected through a blood draw. In a lab, his T-cells were genetically modified to produce special receptors on their surfaces, called chimeric antigen receptors (CARs). CARs allow the T-cells to recognize a specific protein (antigen) on tumor cells. After infusion back into Legaspi’s body, the CAR T-cells multiplied and, with guidance from their engineered receptor, recognized and killed his leukemia cells because they harbored that particular antigen.
“We’re so happy to see Robert respond this quickly and positively to the CAR T-cell therapy. This was a first for us in San Diego and it was exciting to see it work,” said Ball, who also directs the Bone Marrow Transplant Program at Moores Cancer Center. “We are motivated to provide this therapy option to more patients, but we’re also cautious, as CAR T-cell therapies are still in early-phase clinical trials, and there have been serious side effects, though generally manageable, and we do not know the long-term effects of the treatment.”
It took about two months after the one-time CAR T-cell infusion to send Legaspi’s leukemia into remission — a far shorter time than the two years of chemotherapy and radiation to which he was accustomed. He said that getting his CAR T-cells back through IV infusion wasn’t at all uncomfortable — in fact, he fell asleep out of boredom — and he only experienced side effects for about a week. Legaspi’s only appointments these days are for follow-up blood tests and an occasional bone marrow biopsy.
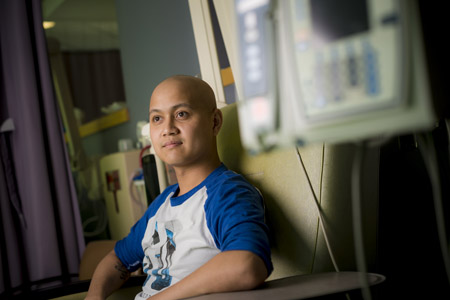
Robert Legaspi sits in a chair in the infusion center at Moores Cancer Center at UC San Diego Health, where he once underwent chemotherapy and most recently received CAR T-cell therapy. Photo courtesy of Erik Jepsen, UC San Diego Publications.
Now, Legaspi feels he has the time and energy to begin building a career. He is going to school to earn his associated degree in nursing. After spending much of his life in hospitals, Legaspi wants to give back.
“If I end up as nurse working with cancer patients, I’ll know what they’re going through and I’ll be able to help them get through it by sharing all the tips and tricks I’ve learned,” he said.
Legaspi is also taking up new hobbies — biking and hiking. He enjoys climbing Cowles Mountain in San Diego’s Mission Trails Regional Park and is now working his way up to more difficult trails.
“I feel like the Hulk now, but instead of gamma radiation like that which made him so strong, I have extra-powerful T-cells — I feel immune to anything!” he said.
About Moores Cancer Center at UC San Diego Health
Moores Cancer Center is one of only 45 National Cancer Institute-designated comprehensive cancer centers in the country. It is also the first and only San Diego-based member of the National Comprehensive Cancer Network, an alliance of the world’s leading cancer centers, and is certified by the Quality Oncology Practice Initiative (QOPL), the leading quality program of the American Society of Clinical Oncology.
For adults newly diagnosed with cancer, treatment at an NCI-designated comprehensive cancer center means superior survival and recovery rates due to the fullness of care, diverse medical, surgical, and radiation oncology sub-specialties, access to breakthrough clinical trials, advanced supportive care and utilization of exacting quality metrics.
Patients have access to therapies, surgeries and clinical trials not offered in community settings. Moores Cancer Center currently operates more than 170 open treatment trials. These investigational therapies include advanced, highly personalized stem cell-based approaches and immunotherapies that leverage the inherent healing powers of the human body.
In late-2016, UC San Diego Health will open Jacobs Medical Center, a 245-bed, 10-story facility where patients with cancer will have access to the Pauline and Stanley Foster Pavilion for Cancer Care, a space dedicated to specialized oncology, and to the A. Vassiliadis Family Pavilion for Advanced Surgery where surgical options include minimally invasive approaches, robotics, transplantation and other combinations of 3D technologies and lifesaving techniques that are only found at UC San Diego Health.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



