Getting Rid of Old Mitochondria
Some neurons turn to neighbors to help take out the trash
Published Date
By:
- Scott LaFee
Share This:
Article Content
It’s broadly assumed that cells degrade and recycle their own old or damaged organelles, but researchers at University of California, San Diego School of Medicine, The Johns Hopkins University School of Medicine and Kennedy Krieger Institute have discovered that some neurons transfer unwanted mitochondria – the tiny power plants inside cells – to supporting glial cells called astrocytes for disposal.
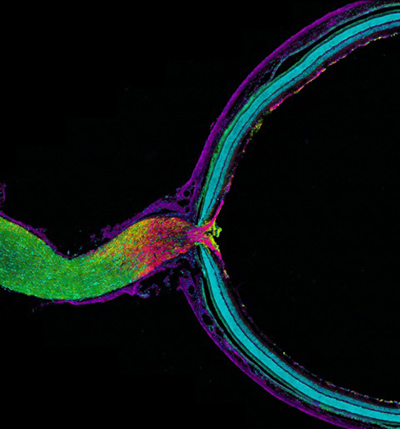
Pictured is mouse optic nerve and retina, responsible for relaying information from the eye to the brain. The tissue has been fluorescently stained to reveal the distribution of astrocytes (yellow), retinal ganglion cell axons (purple), myelin (green) and nuclei (cyan). Retinal ganglion cell axons transfer mitochondria to adjacent astrocytes in the optic nerve head behind the retina. Astrocytes degrade the mitochondria in a process called transmitophagy. Image courtesy of Mark Ellisman, NCMIR, UC San Diego.
The findings, published in the June 17 online Early Edition of PNAS, suggest some basic biology may need revising, but they also have potential implications for improving the understanding and treatment of many neurodegenerative and metabolic disorders.
“It does call into question the conventional assumption that cells necessarily degrade their own organelles. We don’t yet know how generalized this process is throughout the brain, but our work suggests it’s probably widespread,” said Mark H. Ellisman, PhD, Distinguished Professor of Neurosciences, director of the National Center for Microscopy and Imaging Research (NCMIR) at UC San Diego and co-senior author of the study with Nicholas Marsh-Armstrong, PhD, in the Department of Neuroscience at Johns Hopkins University and the Hugo W. Moser Research Institute at Kennedy Krieger Institute in Baltimore.
“The discovery of a standard process for transfer of trash from neuron to glia will most likely be very important to understanding age-related declines in function of the brain and neurodegenerative or metabolic disorders,” Marsh-Armstrong said. “We expect the impact to be significant in other areas of biomedicine as well.”
The researchers looked specifically at the axons of retinal ganglion cells in mice, a type of neuron that transmits visual information from the eye to the brain. The investigation was prompted by observations by Marsh-Armstrong while studying a mouse model of glaucoma that protein products from the retina were accumulating in the optic nerve head (ONH) just behind the eye.
Using a combination of advanced microscopy and molecular techniques developed at the Ellisman and Marsh-Armstrong laboratories, they discovered that damaged mitochondria in retinal ganglion cells were shed at the ONH where ganglion cell axons exit the eye to form the optic nerve leading to the brain. These mitochondria were taken up and degraded by adjacent astrocytes, the most abundant form of glial cell in the vertebrate nervous system and the only cell which bridges between nerve cells and the brain’s blood supply.
The discovery refutes the common assumption that all cells internally isolate, degrade and remove damaged materials – a process generally known as autophagy (Greek for “to self-eat”). When the process involves mitochondria, it’s called mitophagy. The process described by Marsh-Armstrong, Ellisman and colleagues has been dubbed “transmitophagy.”
The surprising findings still leave questions to be answered. For example, do the mitochondria removed at the ONH originate only from the population residing in the long conducting nerve fibers from the eye to the brain or are some actively transported from the retina itself?
Ellisman said the findings could potentially improve understanding – and perhaps eventually the treatment – of diverse disorders. “Mitochondria play prominent roles in the health of axons, which are fundamental to connecting neurons and transmitting information. It should be a priority to further explore what happens in transmitophagy and whether defects in this phenomenon contribute to neuronal dysfunction or disease.”
Co-authors include Chung-ha O. Davis, Elizabeth A. Mills and Judy V. Nguyen, The Johns Hopkins University School of Medicine and Kennedy Krieger Institute; Keu-Young Kim, Eric A. Bushong, Daniela Boassa, Tiffany Shih, Mira Kinebuchi and Sebastien Phan, National Center for Microscopy and Imaging Research, UCSD; Yi Zhou, Kennedy Krieger Institute; Nathan A. Bihlmeyer and Yunju Jin, The Johns Hopkins University School of Medicine.
Funding for this research came, in part, from the National Institutes of Health (grants R01 EY022680 and R01 EY019960), the International Retinal Research Foundation, the Glaucoma Research Foundation, the Melza M. and Frank Theodore Barr Foundation, the National Center for Research Resources (grant 5P41RR004050), the National Institute on Drug Abuse Human Brain Project (grant DA016602), the National Institute of General Medical Sciences (grants 5R01GM82949 and 5P41GM103412-25), NIGMS training grant 5T32GM07814 and the National Science Foundation (grant DGE-1232825).
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.