Gene Mutations Cause Massive Brain Asymmetry
Discovery could help lead to prevention of radical surgery in rare childhood disease
By:
- Scott LaFee
Published Date
By:
- Scott LaFee
Share This:
Article Content
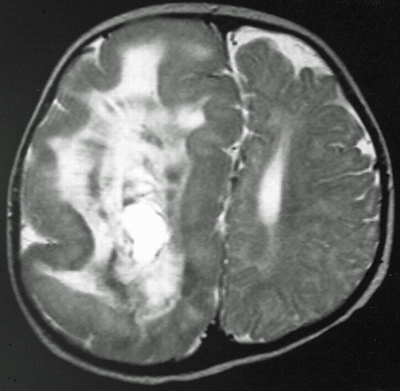
Hemimegalencephaly
Hemimegalencephaly is a rare but dramatic condition in which the brain grows asymmetrically, with one hemisphere becoming massively enlarged. Though frequently diagnosed in children with severe epilepsy, the cause of hemimegalencephaly is unknown and current treatment is radical: surgical removal of some or all of the diseased half of the brain.
In a paper published in the June 24, 2012 online issue of Nature Genetics, a team of doctors and scientists, led by researchers at the University of California, San Diego School of Medicine and the Howard Hughes Medical Institute, say de novo somatic mutations in a trio of genes that help regulate cell size and proliferation are likely culprits for causing hemimegalencephaly, though perhaps not the only ones.
De novo somatic mutations are genetic changes in non-sex cells that are neither possessed nor transmitted by either parent. The scientists’ findings – a collaboration between Joseph G. Gleeson, MD, professor of neurosciences and pediatrics at UC San Diego School of Medicine and Rady Children’s Hospital-San Diego; Gary W. Mathern, MD, a neurosurgeon at UC Los Angeles’ Mattel Children’s Hospital; and colleagues – suggest it may be possible to design drugs that inhibit or turn down signals from these mutated genes, reducing or even preventing the need for surgery.
Gleeson’s lab studied a group of 20 patients with hemimegalencephaly upon whom Mathern had operated, analyzing and comparing DNA sequences from removed brain tissue with DNA from the patients’ blood and saliva.
“Mathern had reported a family with identical twins, in which one had hemimegalencephaly and one did not. Since such twins share all inherited DNA, we got to thinking that there may be a new mutation that arose in the diseased brain that causes the condition,” said Gleeson. Realizing they shared the same ideas about potential causes, the physicians set out to tackle this question using new exome sequencing technology, which allows sequencing of all of the protein-coding exons of the genome at the same time.
The researchers ultimately identified three gene mutations found only in the diseased brain samples. All three mutated genes had previously been linked to cancers.
“We found mutations in a high percentage of the cells in genes regulating the cellular growth pathways in hemimegalencephaly,” said Gleeson. “These same mutations have been found in various solid malignancies, including breast and pancreatic cancer. For reasons we do not yet understand, our patients do not develop cancer, but rather this unusual brain condition. Either there are other mutations required for cancer propagation that are missing in these patients, or neurons are not capable of forming these types of cancers.”
The mutations were found in 30 percent of the patients studied, indicating other factors are involved. Nonetheless, the researchers have begun investigating potential treatments that address the known gene mutations, with the clear goal of finding a way to avoid the need for surgery.
“Although counterintuitive, hemimegalencephaly patients are far better off following the functional removal or disconnection of the enlarged hemisphere,” said Mathern. “Prior to the surgery, most patients have devastating epilepsy, with hundreds of seizures per day, completely resistant to even our most powerful anti-seizure medications. The surgery disconnects the affected hemisphere from the rest of the brain, causing the seizures to stop. If performed at a young age and with appropriate rehabilitation, most children suffer less language or cognitive delay due to neural plasticity of the remaining hemisphere.”
But a less-invasive drug therapy would still be more appealing.
“We know that certain already-approved medications can turn down the signaling pathway used by the mutated genes in hemimegalencephaly,” said lead author and former UC San Diego post-doctoral researcher Jeong Ho Lee, now at the Korea Advanced Institute of Science and Technology. “We would like to know if future patients might benefit from such a treatment. Wouldn’t it be wonderful if our results could prevent the need for such radical procedures in these children?”
Co-authors are My Huynh, department of Neurosurgery and Psychiatry and Biobehavioral Sciences, Mattel Children’s Hospital, Geffen School of Medicine, UCLA; Jennifer L. Silhavy, Tracy Dixon-Salazar, Andrew Heiberg, Eric Scott, Kiley J. Hill and Adrienne Collazo, Institute for Genomic Medicine, Rady Children’s Hospital, UC San Diego and Howard Hughes Medical Institute; Sangwoo Kim and Vineet Bafna, Department of Computer Sciences, Jacobs School of Engineering, UC San Diego; Vincent Furnari and Carsten Russ, Institute for Medical Genetics, Cedars-Sinai Medical Center, Los Angeles and Department of Pediatrics, Geffen School of Medicine, UCLA; and Stacey B. Gabriel, The Broad Institute of MIT and Harvard, Cambridge.
Funding for this research came, in part, from the Daland Fellowship from the American Philosophical Society, the National Institutes of Health (grants R01 NS038992, R01 NS048453, R01 NS052455, R01 NS41537 and P01 HD070494), the Simons Foundation Autism Research Initiative and the Howard Hughes Medical Institute.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



