Computational Models Move Researchers Closer to Tuberculosis Vaccine
Expanse Supercomputer Spotlights Role of Unstable Cells in Response to Infection
Published Date
Article Content
According to a 2021 World Health Organization report, the global COVID-19 pandemic caused an increase in tuberculosis (TB) deaths – 1.5 million in 2020 versus 1.4 million in 2019 – due to a lack of efficient diagnosis and treatment. Since TB has been the number one cause of death from infectious disease in the world for centuries, University of Michigan (UM) Medical School Professor Denise Kirschner and colleagues have been working to better understand the disease by using supercomputers like Expanse at the San Diego Supercomputer Center at UC San Diego.
Recently, Kirschner and the UM team published their most recent findings in the Frontiers in Immunology journal.
“Even during this COVID-19 pandemic, TB remains the leading cause of death by infectious disease around the world,” said Kirschner, a mathematical biologist. “Until our recent study, a particular group of cells called neutrophils have been somewhat of a black box, but our latest work has allowed us to show the potential role of these cells in the immune response to TB infection.”
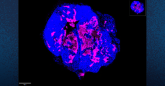
Kirschner explained that neutrophils are unstable cells with short life spans when grown in the lab, but predictive simulations using the Expanse supercomputer allowed her team to use images of the cells inside of a TB granuloma to create high-resolution models illustrating how these mysterious cells react when infected with TB.
“The immune system is made of up of many cells that help respond to an invasion of microbial pathogens and advancements in experimental science gave us imaging of these cells in the granuloma so that we could use that information to inform our model,” she said. “Our new simulations helped us illustrate that there must exist a dual role of neutrophils, which explains why they have been so difficult for us to study and predict their behavior,” she said.
With these new simulations, Kirschner said that the research team now has both mechanisms and information regarding the role of neutrophils in the immune response to infection during tuberculosis. Specifically, their new computational models showed how the host process contributes to immune and drug dynamics – ranging from the molecular level to the entire host.
Why It’s Important
Any scientific progress made on understanding how to improve immunity to TB infection can greatly impact the world.
According to researchers, infection results from inhalation of the bacteria Mycobacterium tuberculosis (Mtb). While TB is treatable, it requires multiple antibiotics taken for more than six months, and the emergence of drug resistant Mtb has strained the current arsenal of effective TB drugs. The situation is critical considering there are nine million new cases of active TB every year and over one million annual deaths.
The pathological hallmarks of TB are lung granulomas, which are dense spherical collections of immune cells that serve to protect the host by isolating bacteria; however, this also provides a niche for bacterial survival and growth. Because granulomas are dense heterogeneous structures, they also present a physical barrier for antibiotic diffusion, slowing drug penetration. These difficulties contribute to the challenge of devising new and more effective treatment strategies for TB: getting the right drugs at the right concentration to the right location to kill the appropriate bacterial subpopulation.
Additionally, while there is a vaccine for TB, known as BCG, it is not used in the U.S. because of its variable efficacy and nullification of available TB tests; a highly effective vaccine still remains elusive though there are currently 10 candidate vaccines in trial.
“Our computational approach and results from our recent study have helped narrow this concerning ‘vaccine design space’ – getting us closer to a globally effective vaccine,” Kirschner said. “Developing and testing these models and using them to make critical predictions about TB treatment and prevention, is a decade-long project and we are hopeful that this current research gets us that much closer to a vaccine that can be used throughout the world.”
How the Expanse Supercomputer Helped
The team’s computational models would not have been possible on a desktop computer – even the most powerful one – as thousands of runs of a sequential simulation model were required to attain the results. And, their complex code required a supercomputer like Expanse, which Kirschner said provided the perfect solution for the supercomputing needs.
“Our code ran well on Expanse, as did our workflow scripts, so this freed up our time to focus on additional research,” she said. “We have received generous time on the Extreme Science and Engineering Discovery Environment (XSEDE) for many years for our TB studies – using supercomputers throughout the U.S.: TACC’s Stampede, SDSC’s Comet and now Expanse.”
This research was supported by grants from the National Institutes of Health, Bill and Melinda Gates Foundation, and the Wellcome Trust. Computations on Expanse were allocated by XSEDE (TG-MCB140228). Data derived from The University of Pittsburgh non-human primate tuberculosis lab of Drs. JoAnne Flynn and Joshua Matilla made this work possible.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.