Chemists Suited to Break Rule, Devise New Chemical Tool
Team of chemistry researchers at UC San Diego develops new method for adding ammonia to molecules
Published Date
Article Content

Graduate and undergraduate students work together in the Schmidt Group lab. Photo by Michelle Fredricks
Just as businesspeople dress up for work, chemists suit up, too. Rather than blazers, browline glasses and Rolex watches, however, working chemists typically wear lab coats, safety goggles and protective gloves. A visit to a chemistry lab at UC San Diego can feel like stepping into a scientific board room. This is the case in Valerie Schmidt’s lab, where “chem suits” monitor bubbling reactions and make tools from things like insulation and tiny rocks.
Schmidt, suited to her trade as an organic chemist specializing in synthesis, catalysis and developing experimental methods, recently published an article in the Journal of the American Chemical Society. The paper outlines her team’s work on developing a new, low-cost method for chemical syntheses involving the use of ammonia.

Graduate student Sam Lardy at work in the Schmidt Group lab. Photo by Michelle Fredricks
Amine Synthesis
An assistant professor in UC San Diego's Department of Chemistry and Biochemistry, Schmidt describes ammonia as a simple molecule that literally feeds the planet. It is used in fertilizers that grow the world’s food supply and provides the nitrogen atoms that are used to biochemically assemble essential components of life, like DNA and proteins. Plant cells are able to break apart ammonia and insert the nitrogen atoms into molecular organic frameworks producing proteins and DNA. These same nitrogen atoms are carried forward up the food chain through metabolism all the way to people.
"Each of us has millions of nitrogen atoms that originally came from ammonia," noted Schmidt.
She explained that plants have evolved to create ammonia from air, called dinitrogen fixation (legumes are plants particularly known for this ability), or to absorb ammonia from the soil in carefully controlled cellular environments. However, in the laboratory, ammonia is trickier. A corrosive, toxic gas with a characteristically strong odor, ammonia is commonly used in cleaning fluids. Chemists currently dissolve it in water to produce an alkaline solution for their experimental reactions.
Schmidt’s novel method, however, offers a solution to using ammonia directly for making new molecules. It allows her and her team to incorporate nitrogen atoms into molecules in a flask, outside the safety of a cell, while maintaining the same effect as if ammonia itself were used.
“Our research results present a new chemical tool in the toolbox of synthetic chemists and may find use in contexts we cannot yet predict,” said Schmidt. “Having the tool is the first step toward using it for future innovations.”
According to Schmidt, all pharmaceuticals, materials and consumer goods are at some level derivatives of the chemical industry. While companies like Merck and Johnson & Johnson disclose the ingredients of the end products consumers buy, the way they make those ingredients is closely guarded and patented.
“This work helps to push forward our frontier of knowledge and builds a new and distinct chemical approach to amine synthesis,” she said.
Markovnikov’s Rule
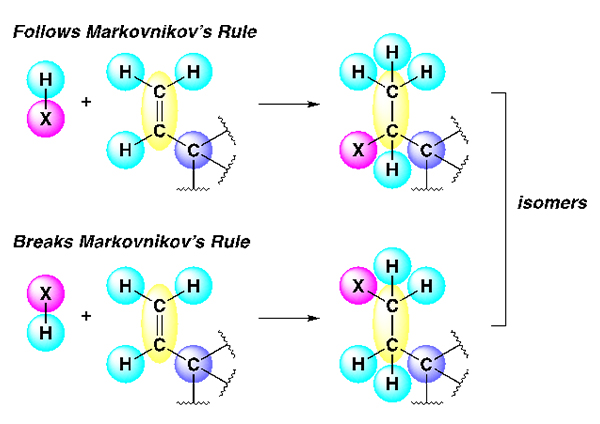
Image depicting Markovnikov’s Rule. Courtesy of Valerie Schmidt
It does this by breaking what’s known in the workaday world of chemists as Markovnikov’s Rule. Vladimir Markovnikov was a Russian chemist from the mid- to late 1800s who developed a rule by which compounds generally abide. When a molecule adds to a carbon-carbon double bond, there are two possible ways for this addition to occur, resulting in two distinct products (or isomers). Markovnikov’s Rule states that the isomer that will form is the one where the hydrogen-atom (light blue circle) will add to the end where there are already two more hydrogen atoms (light blue circles).
“Our new strategy effectively breaks Markovnikov’s Rule by playing a different game—our tactic changes the basic way the molecular components approach each other,” explained Schmidt.
The alternative approach to amine synthesis is mechanistically distinct from other previous methods that achieve the same transformation, according to Schmidt.
“Our approach leverages the thermodynamic properties of the N-hydroxyphthalimide and triethylphosphite reagents to drive the reaction forward by exchanging weak N-O and O-H bonds for stronger O=P and C-H bonds,” explained Schmidt. “We have preliminary data that indicates this approach is more general than for use in alkene hydroamination alone and will be published in the near future.”
Graduate Student Impact
Schmidt credited graduate student Sam Lardy with conducting most of the lab work, including reaction condition optimization, filling out the substrate scope and carrying out isotopic labeling experiments.
“It’s always awe-inspiring to watch a spark of an idea morph into reality and then into a publication,” said Schmidt.
At UC San Diego our research efforts are designed to change the world for the better. The Department of Chemistry and Biochemistry is a vibrant and dynamic interdisciplinary department on campus with close ties and many joint appointments with the Schools of Medicine and Pharmacy and Pharmaceutical Sciences in the Health Sciences, the Scripps Institution of Oceanography, the Jacobs School of Engineering and the Division of Biological Sciences.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.