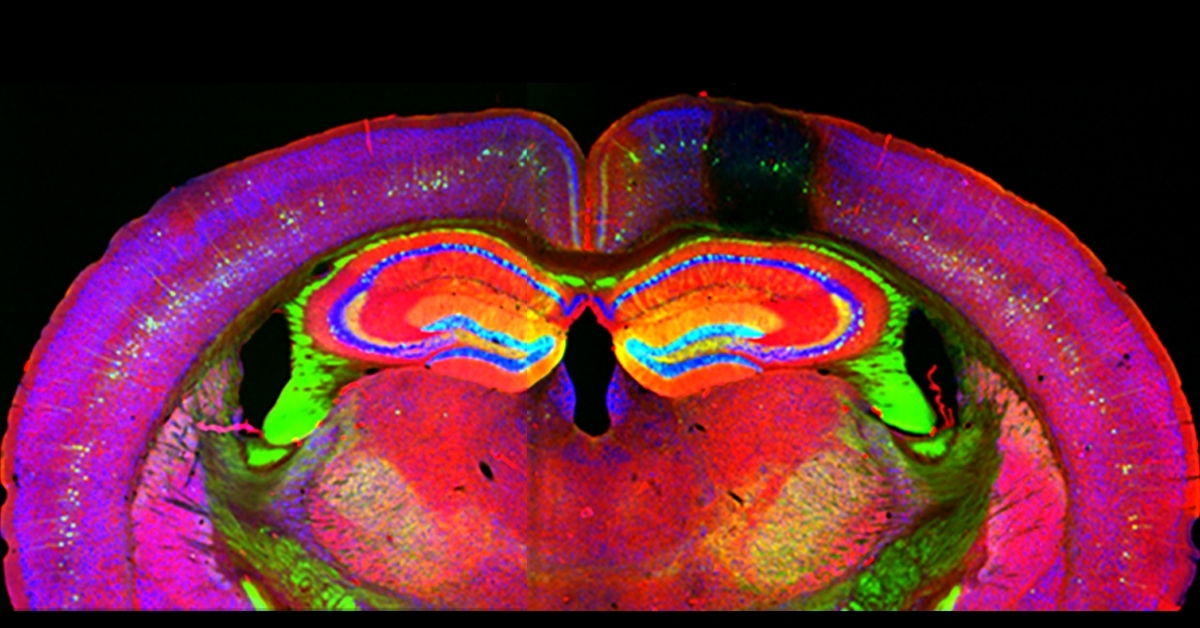
Brain Cells Under Attack Don ‘Body Armor’ Response, Offering New Views of Stroke Damage
Cells within neurons discovered to take ‘pro-survival’ steps to reduce brain tissue injury
Story by:
Published Date
Article Content
University of California San Diego neurobiologists have found that brain cells feature a remarkable ability to shield themselves in situations of severe stress, such as those suffered under stroke conditions.
Treatments for stroke, also known as a “brain attack” since blood flow to the brain is restricted, have largely focused on preventing stroke conditions. Far less is known about core biological functions while such damage takes place.
Research led by Associate Project Scientist Barbara Calabrese and Professor Shelley Halpain in the School of Biological Sciences details what happens within brain cells as they detect threatening conditions. Cells of neurons, the brain’s transmission connectors, can reorganize themselves into a “pro-survival” mode of protection, the researchers found.
“We have discovered that there are processes within neurons that are like putting on body armor,” said Halpain, a faculty member in the Department of Neurobiology. “Detecting stress early and taking steps to respond allows cells to recover and limit damage to the brain.”
Stroke, already a leading cause of death and chronic disability, widened as a public health concern in recent years after experts found that severely ill COVID-19 patients suffer from a higher risk of stroke. During a stroke, or other neurologically threatening situations such as oxygen depletion or traumatic brain injuries, damage to the brain, including neuron swelling, can spread across the brain in waves over subsequent hours and days. Few emergency treatment options exist, ushering the need for a deeper understanding of the underlying biological processes as the damage unfolds. While anticoagulant therapies are regularly used during emergency treatments of stroke, the new findings, published in the journal Nature Communications, offer a novel path to limiting damage to neurons.
“We now have the opportunity to potentially exploit the cell’s own protective mechanisms to try to make their built-in defenses better,” said Halpain. “This may be an alternative way to develop treatments against brain-related diseases and injury. Can we intervene in the cascade of events that worsen damage and reduce the amount of repair needed in the end? How can we rescue the injured but still alive neurons?”
Halpain and her colleagues came across the protective armor phenomenon several years ago, but only recently unlocked an understanding of what it means and how it functions. Initially Halpain assumed the cell’s reorganization was part of the cell’s deterioration under stress damage, and was surprised to find the opposite was true.
Using time-lapse imaging of individual neurons and other techniques, the researchers traced the protective source to actin, a nimble protein involved in various functions within the cell. They discovered an extensive reorganization within the cell of filamentous actin, or F-actin, that occurs under conditions such as stroke and oxygen depletion. They ultimately identified the activation of inverted formin-2 (INF2), an actin binding protein, as the key facilitator of the pro-survival reorganization response, which the researchers now call “actinification.”
“It is remarkable how quickly this actin armor can assemble in response to temporary neuronal stress, and then disassemble itself when the stress disappears,” says Calabrese. “We are eager to learn whether this neuronal armor response occurs during concussions and seizures as well.”
Study coauthor and former UC San Diego Postdoctoral Scholar Andy Shih helped uncover the details by developing a model of a stroke in a single tiny vein or artery of the mouse brain. This “mini-stroke” model allowed the researchers to pick apart the processes unfolding since it featured well-defined borders of the stroke.
“We now have a completely new understanding of how neurons protect themselves from the type of damage that they experience in a stroke—we can now capitalize on that new knowledge to try to move toward preclinical studies.”
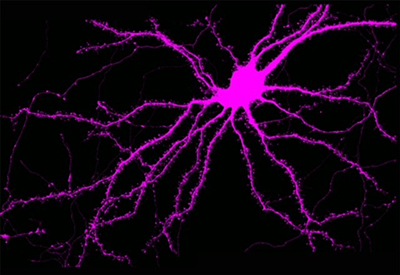
With the new body armor findings in hand, Halpain and her colleagues will delve further into details of the process in animal models, seeking to understand the biochemistry involved in the pro-survival response. They will also study what happens to the synapses and brain circuitry during the protective process. Ultimately, such information could be used as the foundation for potential treatments to make the body armor function more robustly in patients through drug therapies.
“Understanding the cell biology of this response is like the bridge between the biochemistry and animal models,” Halpain said. “We now have a completely new understanding of how neurons protect themselves from the type of damage that they experience in a stroke—we can now capitalize on that new knowledge to try to move toward preclinical studies.”
The authors of the Nature Communications paper include: Barbara Calabrese, Steven Jones, Yoko Shiraishi-Yamaguchi, Michael Lingelbach, Uri Manor, Tatyana Svitkina, Henry Higgs, Andy Shih and Shelley Halpain.
The study was supported by grants from the U.S. National Institutes of Health (grants MH087823, R35 GM140832, NS106138 and NS097775); the Air Force Office of Scientific Research (grant FA9550-18-1-0051); the Defense University Research Instrumentation Program (DURIP) (grant 13107771); the Waitt Advanced Biophotonics Core Facility of the Salk Institute (funding from NIH-NCI CCSG: P30 014195); the Waitt Foundation; and a CZI Imaging Scientist award.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.