Borderzone Breakthrough: A New Source of Cardiac Inflammation
Story by:
Published Date
Article Content
Ischemic heart disease is the most common cause of death in the world. It begins with a “heart attack”, also known as a myocardial infarction (MI), which causes part of the heart to die due to inadequate coronary blood flow. This leads to vigorous inflammation, heart wall remodeling, and heart failure.
Anti-inflammatory drugs have been surprisingly ineffective at preventing heart failure. As a consequence, they are not a routine part of post-MI care. However, it is possible that the most potent molecular and cellular inflammation targets have yet to be discovered.
In the Aug. 28, 2024 issue of Nature, researchers from University of California San Diego in the laboratory of Dr. Kevin King, associate professor of bioengineering and medicine, and a cardiologist at the Sulpizio Cardiovascular Center, report the discovery of a novel mechanism of cardiac inflammation that may expand therapeutic opportunities to prevent heart attacks from becoming heart failure.
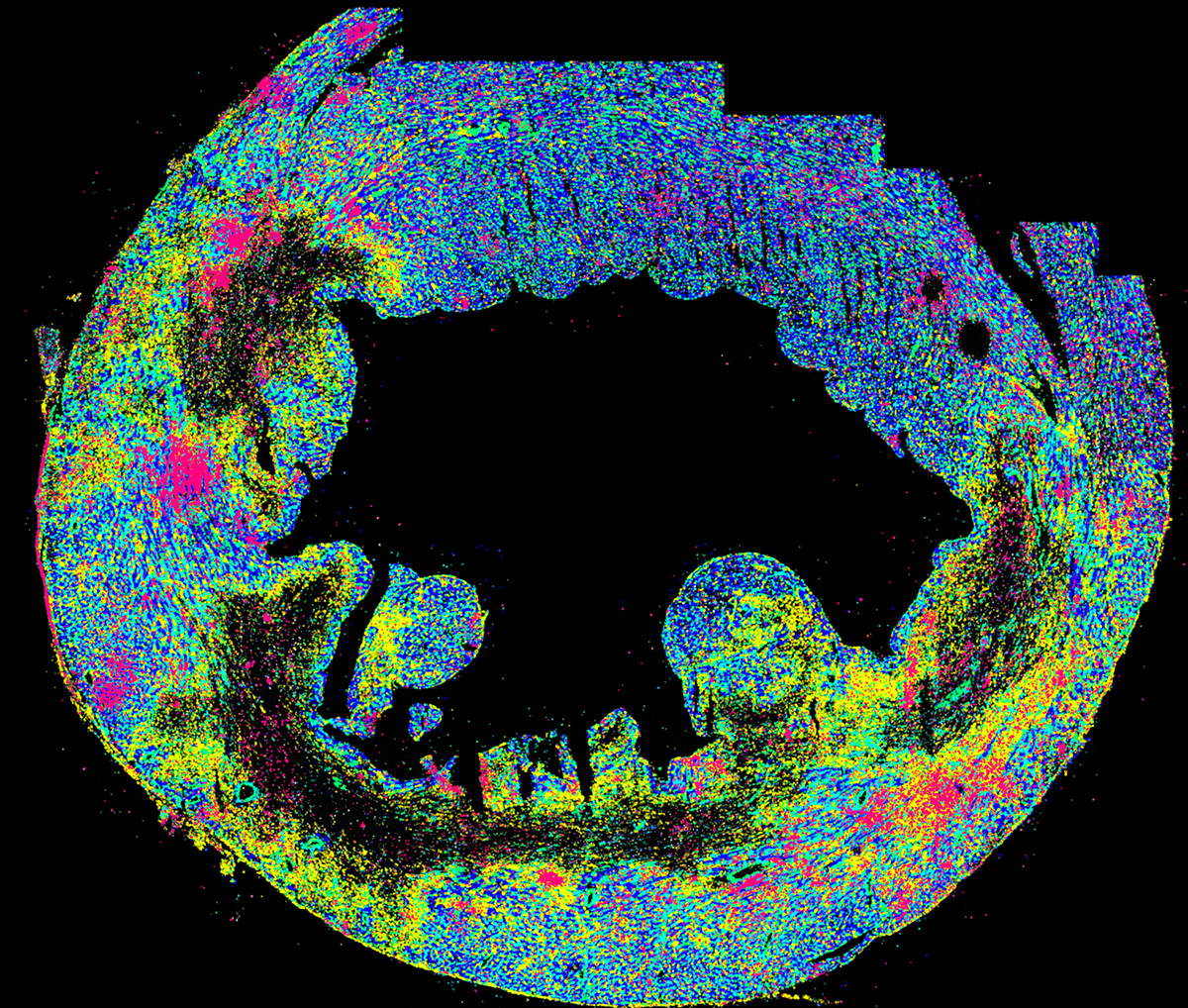
Inflammation after MI is classically credited to professional immune cells like neutrophils and macrophages that infiltrate the infarcted heart and respond to molecules in the debris of dying cells. So the team was surprised when they discovered that the proinflammatory “type I interferon (IFN) response” was activated, not in the infarct where immune cells were concentrated, but instead in the borderzone, surrounding the infarct.
The borderzone has been a fascinating yet understudied area of the infarcted heart. It is where surviving heart muscle cells attempt to stabilize and even proliferate after being disconnected from their dying neighbor cells. Unfortunately, the borderzone has proven a challenging region to study because it is not easily isolated from the rest of the heart. Researchers overcame this obstacle using methods they recently reported based on single cell RNAseq and spatial transcriptomics where cells of the borderzone are recognized based on their patterns of gene expression (https://www.nature.com/articles/s44161-022-00160-3).
To determine which cell type initiates borderzone inflammation, the team created a library of conditional knockout mice, each unable to initiate IFN signaling in a different cell type. To their surprise, heart muscle cells called cardiomyocytes emerged as the dominant initiators of borderzone IFN signaling. They found that mechanically stressed cardiomyocytes in the borderzone frequently suffered nuclear envelope rupture, which allowed escape of nuclear DNA and sensing by cytosolic DNA sensors, leading to activation of IFN signaling. This in turn caused mechanical weakening of the heart wall and made it vulnerable to dilation, thinning, and rupture, providing a mechanistic explanation for the team’s previous reported observation that mice lacking IFN responses exhibited improved survival after MI (https://www.nature.com/articles/nm.4428).
“In the hospital, we care for patients with heart attacks and heart failure every day. New therapeutic targets for MI with the potential to prevent development of heart failure are incredibly important, said Dr. King, senior author of the study an on the faculty in the Shu Chien Gene Lay Department of Bioengineering and the Division of Cardiology at UC San Diego.
Many questions remain, however the newly reported findings suggest that limiting mechanical stress at the borderzone, inhibiting DNA sensing, and preventing type I IFN signaling may represent new opportunities for patients to avoid development of heart failure after MI.
Funding support for the study came, in part, from the NIH DP2 New Innovator Award.
Nature paper: Spatially clustered type I interferon responses at injury borderzones
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



