By:
- Heather Buschman
Published Date
By:
- Heather Buschman
Share This:
When the Language of Cells is Interrupted
New Cell Signaling Center supports research on the most fundamental of cellular function—and development of therapeutics that target them
As Alexandra Newton took the stage late last year to give an invited lecture at the University of Dundee in Scotland, the event host introduced her as hailing from San Diego, “the other top cell signaling community in the world.”

Alexandra Newton (right) and members of her lab at UC San Diego School of Medicine (pictured pre-COVID-19 pandemic.
Until that moment, Newton hadn’t thought of it that way. Yet it struck her as true.
“We probably don’t fully appreciate our own depth of expertise because it’s our normal,” said Newton, Distinguished Professor in the Department of Pharmacology at UC San Diego School of Medicine. “So, I figured if this university has a center dedicated to cell signaling, surely UC San Diego should as well.”
And so Newton, with colleague Jin Zhang, professor of pharmacology, launched the Cell Signaling Center at UC San Diego. Their goal is to unite researchers passionate about understanding cell signaling, and leveraging it to treat disease.
Cell signaling: the language of cells
Newton describes cell signaling as the language cells use to communicate among themselves and with the outside world. Say, a signal knocks on a cell’s outer door, so to speak—insulin, for example. The signal first needs to get the message inside the cell, then trigger a change in behavior, such as taking up glucose in this example, or growing, dividing or secreting.
That cascade of events is known as cell signaling, and it’s a language many scientists want to speak so that they can better understand the many ways it can go awry.
“If I talked super-fast or way too slowly, you’d have trouble understanding me,” Newton said. “Similarly, a change in cell signaling can lead to miscommunication, and ultimately to disease.”
Within the complex language of cells, there are a few commonly used “phrases”—phosphorylation is one key example. In phosphorylation, enzymes called kinases add phosphate groups to proteins and other enzymes called phosphatases remove them, changing protein conformation or activity back and forth like a switch. Whether or not a phosphate group is present in a particular place and time can drastically change cell behavior.
Cell signaling malfunctions can lead to a variety of diseases. For example, a sped-up kinase in one pathway might allow cells to divide out of control, leading to cancer. A slowdown of the same kinase may contribute to untimely cell death, as might occur in neurodegenerative diseases, such as Alzheimer’s.
Drug discovery
If we know what has gone wrong, we are in a better position to fix it, Newton said.
“Let’s say you are repeatedly inserting a wrong word in your sentences,” Newton said. “We need to find the wrong word and replace it with the correct word—that’s what we’re doing with targeted therapeutics.
“Often, very small changes are sufficient to disrupt the normal balance of cell signaling. If just a few atoms in one amino acid, the building block of proteins, are off in a kinase, it could cause the enzyme to start spitting out phosphate groups 30 percent faster, changing the behavior of the cell, causing Alzheimer’s or some other disease, and we need therapeutic drugs to slow it down.”

Graduate students in Alexandra Newton’s lab hold a model of Protein Kinase C (pictured pre-COVID-19 pandemic).
There are more than 500 protein kinases operating in our cells, and many current and experimental drugs work by inhibiting these enzymes. One example is the drug imatinib (brand name Gleevec), a kinase inhibitor that has been a game-changer in treating some types of leukemia.
For decades, many researchers had also attempted to develop drugs that inhibit Protein Kinase C (PKC), Newton’s favorite cell signaling molecule, as a means to treat cancer. But in 2015, Newton’s team reversed this 30-year paradigm when they reported evidence that PKC actually suppresses, rather than promotes, tumors. Their work implied that anti-cancer drugs would actually need to do the opposite—boost PKC activity.
“PKC enzymes are the perfect example of the need to understand the complex biochemistry of our target molecules before we start targeting them in therapies,” Newton said.
UC San Diego’s cell signaling legacy
UC San Diego’s global reputation as a leader in cell signaling research grew out of decades of discoveries.
Susan Taylor, professor in the Department of Pharmacology and Department of Chemistry and Biochemistry, is best known for solving the first crystal structure of a protein kinase (PKA, a relative of PKC) in 1991. The structure revealed how kinases are shaped, how their various pieces come together and how they perform their enzymatic reactions.
“I’ll never forget sitting as a group in the San Diego Supercomputer Center one afternoon, looking at a screen together and seeing PKA in 3D—it was so amazing to realize that we were the only people in the world to see the structure, and to know it was just the beginning of a big family of enzymes,” Taylor said.
PKA is still considered the “poster child” for kinases today.
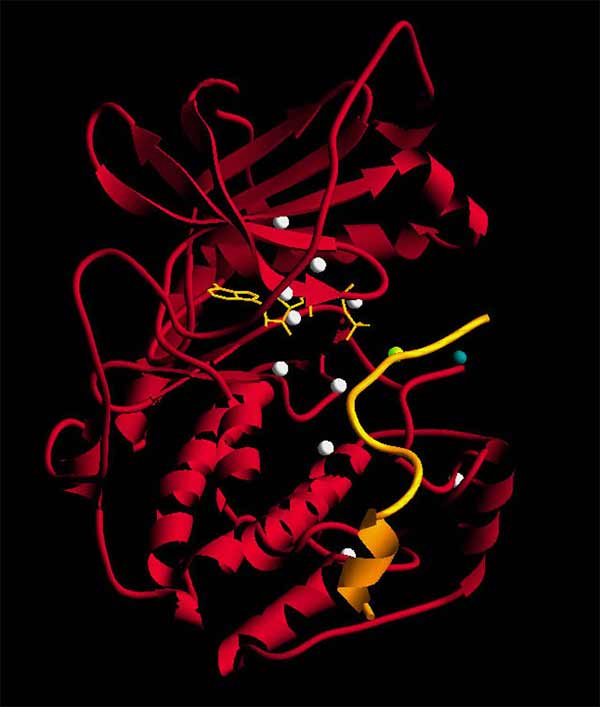
The crystal structure of Protein Kinase A (PKA), shown here, was first solved by Susan Taylor and team in 1991. It’s still considered the gold standard for kinase structures today.
Taylor originally came to UC San Diego as a postdoctoral researcher in 1971, training in the lab of biochemist Nathan Kaplan. There, she worked alongside a number of talented, eager scientists, including graduate student J. Craig Venter and fellow postdoc Jack Dixon.
Venter went on to lead the effort to sequence the human genome. He is currently CEO of the J. Craig Venter Institute.
Dixon became the first to clone, express and characterize a number of phosphatases (the enzymes that do the opposite of kinases—remove phosphate groups). He also discovered the substrate of the tumor-suppressing enzyme PTEN, which shares some structural similarities with the phosphatases he studied. Dixon’s work suggested cell signaling pathways that could be targeted to treat cancers lacking PTEN. He is now Distinguished Professor of Pharmacology, Cellular and Molecular Medicine, and Chemistry and Biochemistry at UC San Diego, where his team is studying a largely overlooked family of secreted kinases.
In the late 1970s, Taylor also taught Dr. Brian Druker when he was an undergraduate and then a medical student at UC San Diego—before he went on to help develop the kinase-inhibitor drug Gleevec, the first FDA-approved therapeutic that works by specifically targeting a molecular defect unique to cancer cells, making it more effective and less damaging to healthy cells.
In addition to kinases and phosphatases, UC San Diego School of Medicine is home to many experts on G-protein coupled receptors (GPCRs), molecules that span cell membranes, where they transmit messages between cells and their environments.
Like kinases, many therapeutic drugs work by influencing GPCRs. Joan Heller Brown, Distinguished Professor of Pharmacology, for example, is known for fundamental contributions to the understanding of GPCRs and how they regulate cell growth and survival in healthy and various disease states, while Dr. Paul Insel, Distinguished Professor of Pharmacology and Medicine, is an expert in the genomics of GPCRs. Silvio Gutkind, who recently succeeded Heller Brown as chair of the Department of Pharmacology, and team are developing innovative anti-cancer prevention and treatment options that work by targeting GPCRs.
Much of today’s cell signaling research relies heavily on leading-edge tools that have grown out of the work of late Nobel Laureate and UC San Diego Professor Roger Tsien—tools that allow researchers to “eavesdrop” on cellular conversations.
Tsien, recruited to UC San Diego in 1989, shared the 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein (GFP), which allows some jellyfish to glow. While colleagues discovered and isolated the GFP gene, Tsien found ways to tweak its makeup, making it glow more brightly and consistently. Then, he created a full color palette of fluorescent proteins that scientists could use to track the molecular goings-on within a cell.
GFP and its many colorful cousins (with names like mCherry and mOrange) quickly became indispensable tools in life sciences labs around the world. Tsien, who died in 2016, often called these fluorescent tags “molecular spies” because they allow researchers to listen in on cellular communications, to watch molecules interact in real-time and ask questions once thought impossible.
With support from Bristol Meyers Squibb, the new Cell Signaling Center is proud to offer an Annual Roger Tsien Cell Signaling Distinguished Lecture Series in his honor.
Cell signaling today—and tomorrow
Today, several of Tsien’s trainees, including Zhang, Nathan Shaner, associate professor of neurosciences, and others are carrying on his legacy at UC San Diego School of Medicine. Shaner, original developer of the GFP-related “fruit series,” continues to engineer and optimize fluorescent proteins for use with newer imaging techniques. Zhang has devised a new set of fluorescent biosensors.
“Our biosensors are unique in that we put in the DNA and cells assemble them for us,” Zhang said. “They allow us to follow a molecule of interest in a live cell, and see what it does, how, when and where.”
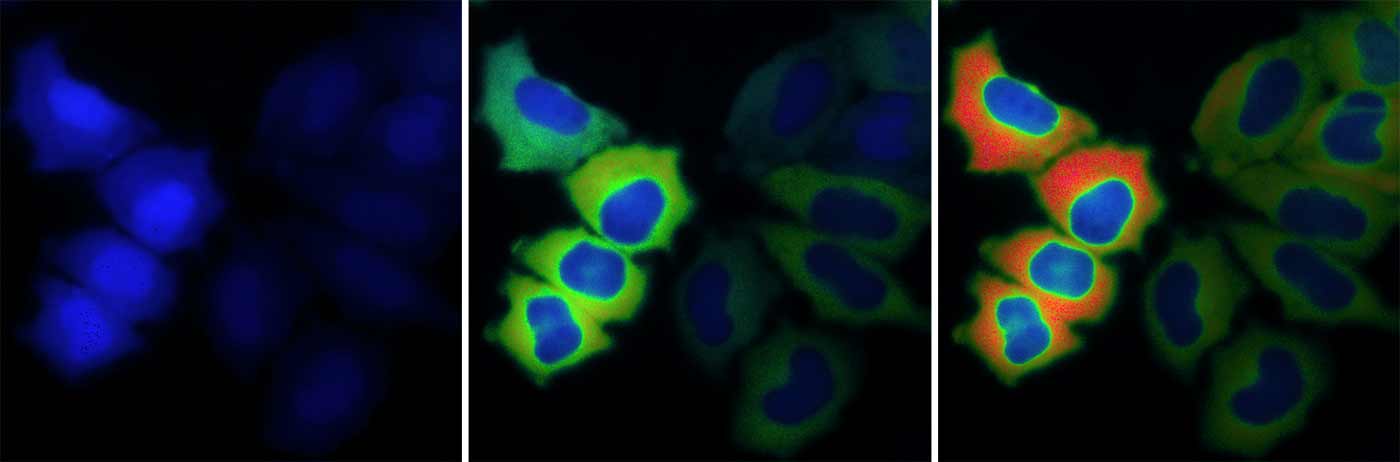
Ultrasensitive biosensors developed by Jin Zhang and team help scientists track kinase activity in live mice.
Hardly a week goes by in which there isn’t a new cell signaling study by UC San Diego School of Medicine researchers published in top scientific journals. In just the last few months:
- Michael Karin and colleagues detailed how excessive fructose metabolism, led by an enzyme called fructokinase, reduces production of proteins that maintain the gut barrier, ultimately leading to chronic inflammation associated with non-alcoholic fatty liver disease (NAFLD).
- Pradipta Ghosh and team discovered that a molecule called GIV acts as a brake on macrophages, keeping them in check. The researchers can also mimic GIV as a means to reduce inflammation in cases where it’s detrimental, such as in sepsis and colitis.
- Samara Reck-Peterson, Andres Leschziner, Elizabeth Villa, Taylor and others used leading-edge imaging technologies known as cryo-electron tomography and cryo-electron microscopy to view a protein called LRRK2 in its natural environment within cells, describe its structure at a level previously unseen, and understand its role in the transports of cellular cargo. Mutations in LRRK2 are thought to be a cause of Parkinson’s disease, and it’s the target of many drug development efforts.
When thinking about the future of the cell signaling field, Taylor said she’s perhaps most excited about recent leaps in imaging capabilities in the past few years, especially when combined with computational and engineering expertise.
For example, researchers were (virtually) lining up to collaborate with new assistant professor Johannes Schöneberg after he presented his work in quantitative 4D imaging at a recent meeting. His technology can, for example, fly viewers through the mitochondria in a live human brain organoid grown in a lab.
“People across disciplines have always worked together well here at UC San Diego, even from the very beginning, and that has been enhanced by many cross-campus joint appointments—like both Johannes Schöneberg and Roger Tsien, who held faculty appointments in both the School of Medicine’s Department of Pharmacology and the Department of Chemistry and Biochemistry on the main campus,” Taylor said.
That collaborative spirit is the basis for the new Cell Signaling Center. According to Zhang, the center will act as a hub for researchers in the field to better connect with each other, as well as a one-stop-shop for local biopharmaceutical companies and colleagues around the world looking for the types of experts and resources that UC San Diego can provide.
“Together, we are taking not a sledgehammer approach to treating disease, but a precise fine-tuning of cell signaling processes to correct their mis-regulation in disease,” Zhang said. “That’s the basis of precision, or personalized, pharmacology, and it starts with the kind of work we’re doing here at UC San Diego.”
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



