We Need a Staph Vaccine: Here’s Why We Don’t Have One
Research from UC San Diego explains the clinical failure of dozens of candidate vaccines for one of the most common human infections; it also suggests a way to fix the problem
Story by:
Published Date
Story by:
Topics covered:
Share This:
Article Content
Staphylococcus aureus (SA) is an extremely common bacterial infection; about 30% of people have colonies of SA living in their nose. SA is often harmless, but it is also a leading cause of hospital-acquired and community-associated infections. A vaccine for SA would be a game-changer for public health, but for decades, all vaccine candidates for SA have failed in clinical trials despite successful preclinical studies in mice. Researchers at University of California San Diego School of Medicine have finally explained why.
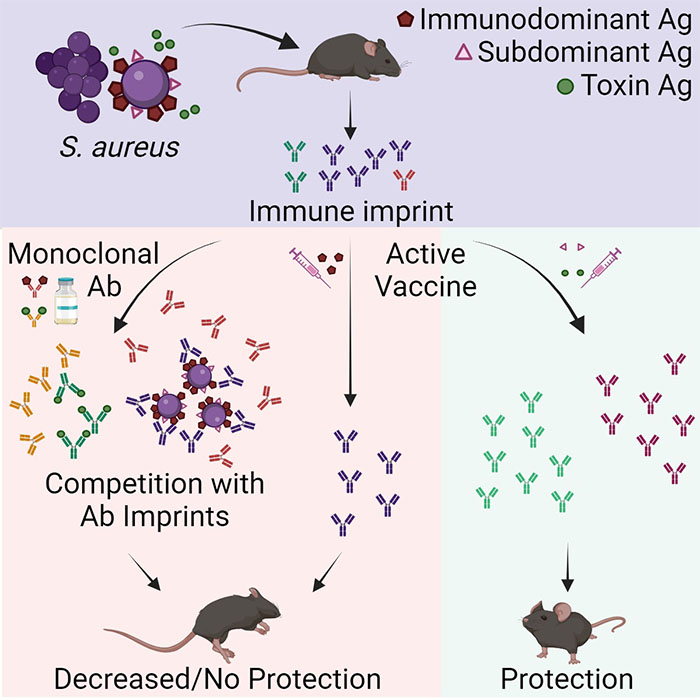
In a new study, published January 16, 2024 in Cell Reports Medicine, they tested a new hypothesis that SA bacteria can trick the body into releasing non-protective antibodies when they first colonize or infect humans. When the individual is later vaccinated, these non-protective antibodies are preferentially recalled, making the vaccine ineffective.
SA has a unique relationship with humans. While it causes many dangerous health complications, including wound and bloodstream infections, the bacterium is also a normal part of the healthy human microbiome, where it lives peacefully in the nose and on the skin.
“SA has been with humans a long time, so it’s learned how to be part-time symbiont, part-time deadly pathogen,” said senior author George Liu MD, PhD, professor in the Department of Pediatrics at UC San Diego School of Medicine. “If we’re going to develop effective vaccines against SA, we need to understand and overcome the strategies it uses to maintain this lifestyle.”
The immune system releases protective antibodies in response to molecules it suspects are foreign, called antigens. These antibodies are then saved in the immune system’s memory, so the next time the immune system encounters that same antigen, it will generally recall its earlier immune response rather than mount a brand-new attack.
“This is an effective system for conferring long-term protection against pathogens, but it only works when the initial immune response to that pathogen was actually protective,” said co-lead author JR Caldera, PhD, who completed his doctoral research in the Liu Lab. “What sets SA apart is that the bacteria themselves have ways of evading the immune system from the moment they encounter us, and these evasive strategies are only reinforced by vaccination.”
While SA vaccines have unilaterally failed in clinical trials, they generally do well in preclinical studies of mice. In order to figure out why this is, the researchers collected blood serum from healthy volunteers, quantifying and purifying the anti-SA antibodies present in the samples. They then transferred these antibodies to mice to explore how protective they were against SA on their own, as well as how they influenced the efficacy of several clinically-tested SA vaccine candidates.
“SA has been with humans a long time, so it’s learned how to be part-time symbiont, part-time deadly pathogen. If we’re going to develop effective vaccines against SA, we need to understand and overcome the strategies it uses to maintain this lifestyle.”
The researchers found that the vaccines were ineffective in mice that had been given human anti-SA antibodies, as well as mice that had been previously exposed to SA. However, in mice that had never been exposed to either SA or human antibodies, the vaccines worked. Unlike previous mouse studies of SA vaccines, the researchers’ results were consistent with those of failed clinical trials, suggesting that their experimental model could help predict the clinical success of SA vaccines while they are still being tested in preclinical mouse studies.
Further, they found that specific antibodies were to blame for the effect they observed. The antibodies that attack the cell walls of SA bacteria, which are the basis for most current SA vaccines, didn’t protect the mice against SA. By contrast, antibodies that target the toxins produced by SA were able to successfully neutralize them.
“One pathogen can have many different antigens that the immune system responds to, but there is a hierarchy as far as which antigen is dominant,” said co-lead author Chih Ming Tsai, PhD, a project scientist in the Liu Lab. “Most vaccines are based on the dominant antigen to trigger the strongest possible immune response. But our findings suggest that for SA, the rules are different, and it is more beneficial to target so-called subdominant antigens, which triggered a weak immune response in the first place.”
In addition to exploring the possibility of targeting new antigens with future SA vaccines, the researchers are also interested in exploring the deeper question at play here: why is the natural human immune response to this bacterium so ineffective to begin with?
“Somehow, SA is able to trick our immune system, and figuring out how will help us improve existing SA vaccines and develop new ones,” said Liu. “More broadly, these findings suggest a whole new way of reevaluating failed vaccines, which could have implications well beyond this one bacterium.”
Co-authors of the study include: Desmond Trieu, Cesia Gonzalez, Irshad A. Hajam, Xin Du and Brian Lin at UC San Diego.
This study was funded, in part, by the National Institute of Health (grants R01AI127406, R01AI144694 and R01AI181321).
Disclosures: George Y. Liu, Chih-Ming Tsai and Irshad A. Hajam have received a research grant from Pfizer to study S. aureus vaccines.
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.




