UC San Diego Chemists Boost Future of New Energy
Research with ultrafast light featured in TV’s ‘Big Bang Theory’ presents possibilities for medical devices and scientific training
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
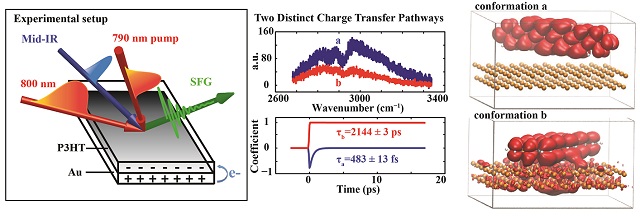
University of California San Diego scientists used ultrafast lasers and supercomputers to develop a new method to probe electron charge transfer at the interface between organic semiconductors and metal surfaces. The UC San Diego research by Department of Chemistry and Biochemistry Assistant Professor Wei Xiong and Professor Francesco Paesani, plus two graduate students and a postdoctoral fellow, marks the first time that this novel direct charge transfer mechanism was measured in energy systems such as solar cells and photovoltaics. These are material sources of "new energy"—naturally replenished resources such as sunlight, wind, rain, waves, geothermal heat — that involve the conversion of light into electricity.
Xiong explained that the team used solid-state ultrafast lasers, “like the one used by Leonard in the Big Bang Theory,” and a scientific-grade, charge-coupled-device camera — similar to popular digital cameras — to conduct the study. Paesani and postdoctoral scholar Cong Huy Pham then used advanced simulation techniques on supercomputers with thousands of central processor units (CPUs) to model the experimental data and provide molecular insights on the charge transfer mechanism.
A charge transfer occurs when a fraction of electronic charges is exchanged between two or more molecules, or different parts of a single large molecule. Examples of materials that exploit charge transfer include solar batteries, solar shades, solar cells integrated into architecture, as well as in materials for power generation in spacecraft. A detailed description of the scientists’ study and methods is outlined in their paper titled, “Ultrafast direct electron transfer at organic semiconductor and metal interfaces,” recently published in Science Advances.
Xiong and doctoral students Yingmin Li and Bo Xiang, who co-first authored the published paper and led the research, observed direct electron transfer between organic polymer semiconductor film and gold substrate. Using ultrafast light — nearly one quadrillionth of a second (0.000000000000001 second) — they developed the new method and used it along with Paesani’s and Pham’s high-level computer simulations to find that when molecules align at certain orientations they allow a novel, more efficient charge transfer mechanism.
“First of all, this new method would allow us to investigate whether the same charge transfer mechanism observed in our system also exists in other energy material interfaces,” explained Xiong about the significance of the research. “Second, this new charge transfer mechanism opens up new possibilities for developing efficient light-harvesting materials and new near-infrared sensors for medical applications.”
According to Xiong, the project was a fantastic journey that began when both Li and Xiang joined his research group, developing an exciting collaboration with the Paesani group.
“The success of this project also demonstrates the importance of training future generations of scientists,” Xiong said. “Furthermore, this work shows how the combination of new experimental and state-of-the-art computational techniques can advance our knowledge in unexplored territory.”
This research was supported by the Defense Advanced Research Projects Agency Young Faculty Award program (D15AP000107), the U.S. Department of Energy, Office of Science, (award DE-FG02-13ER16387), the National Energy Research Scientific Computing Center, which is supported by the Office of Science of the U.S. Department of Energy (contract DEAC02-05CH11231). The gold substrate was fabricated, in part, at the San Diego Nanotechnology Infrastructure of UC San Diego, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the NSF (grant ECCS-1542148). X-ray photoelectron spectroscopy work was performed at the University of California, Irvine Materials Research Institute.
The Department of Chemistry and Biochemistry at UC San Diego is committed to excellence in research, education and service. As part of that mission, it values and promotes equity, fairness and inclusion of diverse members. It is one of three departments, along with mathematics and physics, within the UC San Diego Division of Physical Sciences.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.