Transforming Clinical Recording of Deep Brain Activity with a New Take on Sensor Manufacturing
The new approach allows minimally-invasive, high-resolution recording as deep as 10cm/4in inside the human brain
Story by:
Media contact:
Published Date
Story by:
Media contact:
Share This:
Article Content
Sensors built with a new manufacturing approach are capable of recording activity deep within the brain from large populations of individual neurons–with a resolution of as few as one or two neurons–in humans as well as a range of animal models, according to a study published in the Jan. 17, 2024 issue of the journal Nature Communications. The research team is led by the Integrated Electronics and Biointerfaces Laboratory (IEBL) at the University of California San Diego.
The approach is unique in several ways. It relies on ultra-thin, flexible and customizable probes, made of clinical-grade materials, and equipped with sensors that can record extremely localized brain signals. Because the probes are much smaller than today’s clinical sensors, they can be placed extremely close to one another, allowing for high-resolution sensing in specific areas at unprecedented depths within the brain.
Right now, the probes can record with up to 128 channels, while the state of the art in today’s clinical probes is only 8 to 16 channels. In future, the innovative manufacturing approach the researchers developed can expand the number of channels to thousands per probe, dramatically enhancing physicians’ ability to acquire, analyze and understand brain signals at a higher resolution.
This technology is a first step towards wireless monitoring of patients with treatment-resistant epilepsy for extended periods of time–up to 30 days–as they go about their daily lives. Beyond treatment-resistant epilepsy, the potential applications are much broader, including helping people with Parkinson’s disease, movement disorders, obsessive-compulsive disorder, obesity, treatment-resistant depression, high-impact chronic pain and other disorders.
While the Nature Communications paper reports brain-recording data only, the system has been developed to both record brain activity and provide electrical stimulation to precise locations. In fact, the team is building on previous – and ongoing – work that uses this scalable, thin-film manufacturing approach to create brain-computer interfaces that record activity and deliver therapeutic electrical stimulation to the surface of the brain cortex.
The probes are monolithic, meaning that their individual components are layered on top of one another to create a single, cohesive unit, and do not require manual assembly of additional wires to conduct recordings. The new recording system is both extremely customizable and scalable to manufacture, thanks to thin-film technology derived from the semiconductor and digital-display screen industries. As such, the probes are extremely compact–15 micron thick, or about 1/5th the thickness of a human hair–minimizing the differences between the material properties of the probe and the brain.

“We developed an entirely different manufacturing method for thin-film electrodes that can reach deep brain structures - at a depth that is necessary for therapeutic reasons - enabling reproducible, customizable, and high-throughput production of electrodes but with a high spatial resolution and channel count despite a thinner electrode body. Additionally, the electrode insertion is compatible with existing surgical techniques in the operating room, lowering the barrier for their adoption in clinical procedures,” said UC San Diego electrical engineering professor Shadi Dayeh, the corresponding author on the new paper.
The design, manufacture, experimental testing and analysis of results from this system was performed by a cross-disciplinary team of engineers, surgeons, and medical researchers from UC San Diego; Harvard Medical School and Massachusetts General Hospital; and Oregon Health and Science University.
Dayeh advises two of the three first authors on the paper: UC San Diego postdoctoral researcher Keundong Lee and UC San Diego graduate student researcher Yun Goo Ro. Angelique C. Paulk, also a first author, is a researcher at Massachusetts General Hospital and Harvard Medical School in a group led by neurologist Dr. Sydney Cash.
Toward a 30 day wireless brain-recording system
The kind of system researchers developed is needed in order to identify the very specific regions of the brain that are triggering seizures caused by treatment-resistant epilepsy. To meet this goal, the team is working toward their vision of a brain-monitoring system with sensors both inserted deep within the brain and sensors on the surface of the brain. These sensors will communicate wirelessly with a small computer system in a wireless cap, which a person could wear for extended periods of time. This cap would provide wireless power and the computational infrastructure to capture the brain signals being recorded from a person's brain for 30 days.
“We are currently focused on applying the technology to patients with treatment-resistant epilepsy. The ultimate goal is to advance the system and related required technologies by 2026 to give patients access to a wireless system that allows them to move freely within the hospital environment and then at home, without being tethered to any machinery, while cortical and deep brain structures are monitored continuously for up to 30 days,” Dayeh said.
The system is called the UC San Diego Micro-stereo-electro-encephalography (µSEEG). The technology that is used to create the device can be manufactured at high volume and low cost because it is derived from existing technologies to manufacture digital display screens, an approach that was originally created by the semiconductor industry. This unique manufacturing process also allows for a series of unique features for these depth electrodes (see sidebar).

Experimental subjects
In the new paper, the team reports the functioning of the new system in two human patients. The team also presents data from a series of different animal models including successful recordings from rat barrel cortex in both acute and chronic settings; recording of the somatosensory cortex in an anesthetized pig; and recordings in non-human primates at different depths inside the brain.
The data on the successful functioning of the device in humans were collected, with all proper approvals and consent, during already scheduled tumor-removal surgeries. During an unrelated pause in the surgery, clinicians inserted the new depth probes into brain tissue that was about to be removed.
“In a true test of the translational feasibility of the µSEEG,” the authors write in the Nature Communications paper, referring to the technical term for their device, “we acutely implanted short 64 channel µSEEG electrodes in the middle temporal gyrus in two separate human patient participants undergoing temporal lobe resection for clinical reasons. With each participant, we inserted a single 64 channel short µSEEG device into tissue, which the clinical team determined would be resected.” The recordings lasted 10 minutes and were able to record ongoing spontaneous activity.

Comments from authors on the paper
Dr. Keundong Lee (First author #1), Postdoctoral Fellow at IEBL, UC San Diego
It has been a long journey since 2015 to develop a robust, human-grade depth electrode that can be used in clinical practice. Finally, we have discovered an innovative manufacturing technique to create the µSEEG probe, which can assist with high resolution and minimally invasive diagnosis of epilepsy, and potentially treatment for epilepsy and other indications, in the future.
Beyond epilepsy, continuous monitoring of brain activity at such high resolution could allow us to find biomarkers for other conditions, including perhaps treatment-resistant depression.
Dr. Angelique Paulk (First author #2), Instructor in Neurology at Massachusetts General Research Institute and Harvard Medical School
Our lab has worked with the Dayeh lab for almost a decade to bring this innovative technology to fruition. Around 2018, we tested the laminar version of the UC San Diego microSEEG in two patients at MGH. Through iterative feedback that we and Drs. Sharona Ben-Haim, Ahmed Raslan, Mark Richardson, and Ziv Williams provided to inform probe fabrication, we are now happy with the end result that we feel is much closer to clinical use. We were excited to test the longer version in non-human primates here at MGH and to record the activity of single neurons with these devices.
Dr. Yun Goo Ro (First author #3), PhD graduate from the IEBL, UC San Diego
My research on this electrode was both exciting and challenging as we had to come up with new ways of implementing a scalable electrode with operating principles that are compatible with clinical use. It is very exciting to see my PhD research extended to long electrodes to maximize their clinical impact and I am proud to see the potential of my PhD inventions translate from the benchtop to the bedside.
Dr. Sydney Cash, MD, Professor of Neurology, Massachusetts General Hospital and Harvard Medical School
These new electrode systems are really exciting. They are designed in a way, which can be easily used in the clinical setting yet provide a level of resolution not seen before. There is no question in my mind that this will help us understand both normal brain function and pathology much better and will lead to new ways to help people suffering from epilepsy and a variety of other neurological problems.
Dr. Sharona Ben-Haim, MD, Associate Professor of Neurological Surgery, UC San Diego School of Medicine and Surgical Director of Epilepsy, UC San Diego Health
This new electrode technology is exciting for a large variety of reasons, including its capacity for recording at unprecedented resolution. The future ability of this system to record wirelessly from the brain of epilepsy patients undergoing intracranial EEG evaluation has the potential to dramatically change our current clinical practice. Currently, patients who undergo this type of evaluation remain in the hospital for the duration of the study, where we try to capture where their unique seizures originate during a period of time that typically lasts from 7-21 days. During this time patients are tethered to their hospital beds by the wired cords from the current clinical electrode system. This new technology has the capacity to potentially allow us to send these patients home, freeing them from a long hospital stay, and potentially allowing us to record for longer periods of time and obtain more robust information to help us ultimately treat their seizures with more precision and resolution than previously possible.
Dr. Eric Halgren, Professor of Radiology, Neurosciences, and Psychiatry, UC San Diego
With current electrodes we can only hear the roar of the crowd. The new electrodes will allow us to discern individual voices, and we can begin to learn their language, which is the basic vocabulary of thought.
Dr. Ahmed Raslan, MD, Professorand Vice Chair of Neurological Surgery, Oregon Health and Sciences University
The new depth electrodes combine two unique features: the much higher resolution of recording contacts combined with stimulation capability, which would improve our ability to understand -and potentially change/treat- neural circuits in parts of the brain that are not accessible by surface or penetrating interfaces and at a much higher resolution than current depth electrodes; a strategy that unlocks decades-long trade offs, and, the wireless connectivity which opens the door to recording from humans in an unrestricted environment allowing sampling of various types of behavior. This new electrode is a platform neural interface that can both read and write into the brain in experimental and clinical environments, as such the potential uses and applications are unlimited.
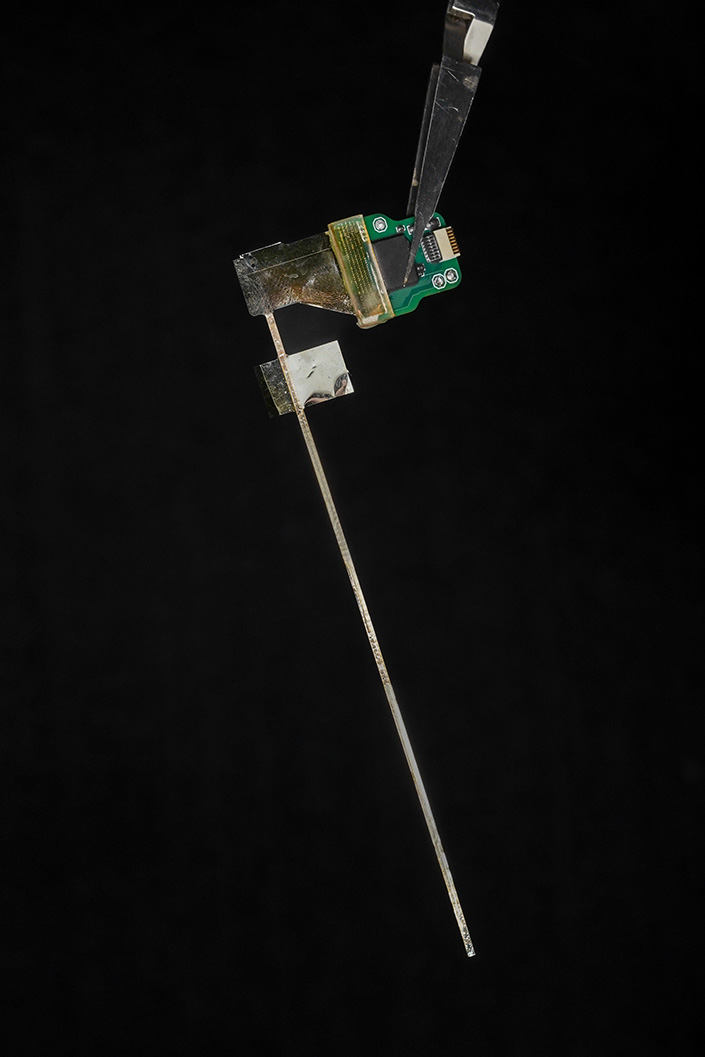
Features of the UC San Diego Micro-stereo-eletro-encephalography (µSEEG)
- The probes can be up to 10 cm in length, allowing for access to structures deep within the brain.
- The probes are incredibly thin: just 15 micron thick, or one-fifth the width of a human hair, and 1.2 millimeters wide
- When inserted into brain tissue, the probe lined with sensors has a thickness that is smaller than technologies currently in clinical use. This smaller thickness means less brain tissue is damaged when the probe is inserted.
- Brain-signal recording electrodes can be placed 60 micrometers apart, which is far closer to each other than technologies currently in clinical use.
- Probes with up to 128 brain-signal-recording channels (electrodes) were demonstrated, compared to 8 to 16 recording channels in today’s broadly used clinical depth electrodes.
- The small size of the electrodes allows for extremely localized brain-signal recording, as precise as the signal coming from the individual activity of one or two neurons. They can also record local field potentials, which is aggregate activity of many neurons within a brain region.
- The electrode sensors are able to record precise areas of the brain over both short and long time periods.
- The electrodes work well: they record brain activity triggered by stimulating a body part, and they record the brain dynamics known to occur during anesthesia.
- The system allowed for simultaneous recording of the cortex of the brain and signals from individual neurons deep within the brain. The researchers were able to correlate the general brain activity to what was happening at the single-neuron level.
- The system allows monitoring the dynamics of brain activity instantaneously, allowing visualization of the propagation of the activity across cortical layers with precision with time.
- Cost-effective, scalable manufacturing of the new system is in direct contrast to the expensive and time-consuming manual assembly required for the systems currently in clinical use. All other known experimental depth electrodes require some amount of manual assembly as well.
Funding
National Institutes of Health BRAIN® Initiative UG3NS123723-01 (S.A.D.); National Institutes of Health BRAIN® Initiative R01NS123655-01 (S.A.D.); National Institutes of Health NBIB DP2-EB029757 (S.A.D.); National Institutes of Health F32 postdoctoral fellowship MH120886-01 (D.R.C); National Science Foundation Award no. 1728497 (S.A.D.); National Science Foundation CAREER no. 1351980 (S.A.D.); National Science Foundation Graduate Research Fellowship Program no. DGE-1650112 (A.M.B.); MGH - ECOR (S.S.C.); K24-NS088568, R01-NS062092 (S.S.C.) Tiny Blue Dot Foundation (to S.S.C. and A.C.P.); National Institutes of Health BRAIN® Initiative K99 NS119291 (K.J.T.), and National Eye Institute R01EY027888 (J.S.P.), William M. Wood Foundation, Bank of America Trustee (J.S.P.).
The depth electrode was fabricated in the UC San Diego Nano3 cleanroom which is part of the San Diego Nanotechnology Infrastructure (SDNI) at UC San Diego, a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the NSF (grant ECCS1542148).
Competing interests
The authors declare the following competing interests: K.L., Y.G.R., and S.A.D. and the University of California San Diego filed a patent application (#63/584,578, pending) for the manufacture of the novel depth electrodes. A.C.P., D.R.C., Y. T., A.M.R., S.B.H., E.H.,
S.S.C., and S.A.D. have competing interests not related to this work including equity in Cortical Science Inc.. S.A.D. was a paid consultant to MaXentric Technologies. A.M.R. has equity and is a cofounder of CerebroAI. A.M.R. received consulting fees from Abbott Inc and Biotronik Inc. The MGH Translational Research Center has clinical research support agreements with Neuralink, Paradromics, and Synchron, for which S.S.C. provides consultative input. The other authors declare that they have no competing interests.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



