Synthetic Membranes Created to Mimic Properties of Living Cells
Published Date
By:
- Kim McDonald
Share This:
Article Content
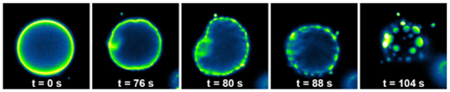
This sequence of fluorescence microscopy images (and the video below) show the spontaneous remodeling of lipids in a synthetic membrane. Images and video by Devaraj Lab, UC San Diego
Biochemists at the University of California San Diego have developed artificial cell membranes that grow and remodel themselves in a manner similar to that of living mammalian cells.
The achievement, detailed in a paper published in this week’s issue of the Proceedings of the National Academy of Sciences, follows the successful design last year in the same laboratory of artificial, or synthetic, cell membranes capable of sustaining continual growth. The two developments now bring the researchers closer to mimicking all of the properties of living mammalian cell membranes with synthetic components.
That’s important because synthetic membranes that accurately mimic the behavior of living mammalian cell membranes could be used by biomedical researchers to develop more effective drugs that target membrane proteins and better understand the chemical changes that occur in dysfunctional membranes during disease.
“While artificial membranes have been used to model the properties of native membranes, previous methods have not been able to mimic lipid membrane remodeling,” said Neal Devaraj, an associate professor of chemistry and biochemistry at UC San Diego who headed the research team for both studies. “In our latest study, we show that reversible chemical reactions can be harnessed to achieve spontaneous remodeling of lipids in synthetic membranes.”
Living cells continually remodel their membranes to change their physical characteristics, a process that can affect the behavior of other biomolecules in the cell membrane.
“Cells use lipid remodeling to respond to their environment and maintain membrane homeostasis or to carry out specific functions such as division and signaling,” said Andrew Rudd, a co-author of the study and graduate student in the Devaraj lab. “Using phospholipid remodeling allows cells to generate new phospholipid species by recycling existing phospholipids instead of making them from scratch. This saves the cell time and energy.”
Devaraj explained that his team’s latest development provides a way for biochemists to better understand the changes that occur in phospholipid membranes during lipid remodeling.
“One exciting application would be to probe the behavior of bound and integral membrane proteins in response to shifts in membrane composition,” explained Roberto Brea, a postdoctoral fellow in the Devaraj lab and the lead author of the study. “Integral membrane proteins are extremely important and common drug targets and we need a way to understand their behavior in lipid bilayers. This is one way to do that.”
Support for the research project was provided by the National Science Foundation and the Human Frontier Science Program.
Share This:
You May Also Like
UC San Diego is Strengthening U.S. Semiconductor Innovation and Workforce Development
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



