Sulfur Signals in Antarctic Snow Reveal Clues to Climate, Past and Future
Anomalous isotopes trace Earth’s ancient atmosphere, past El Niño events and future climate
Published Date
By:
- Susan Brown
Share This:
Article Content
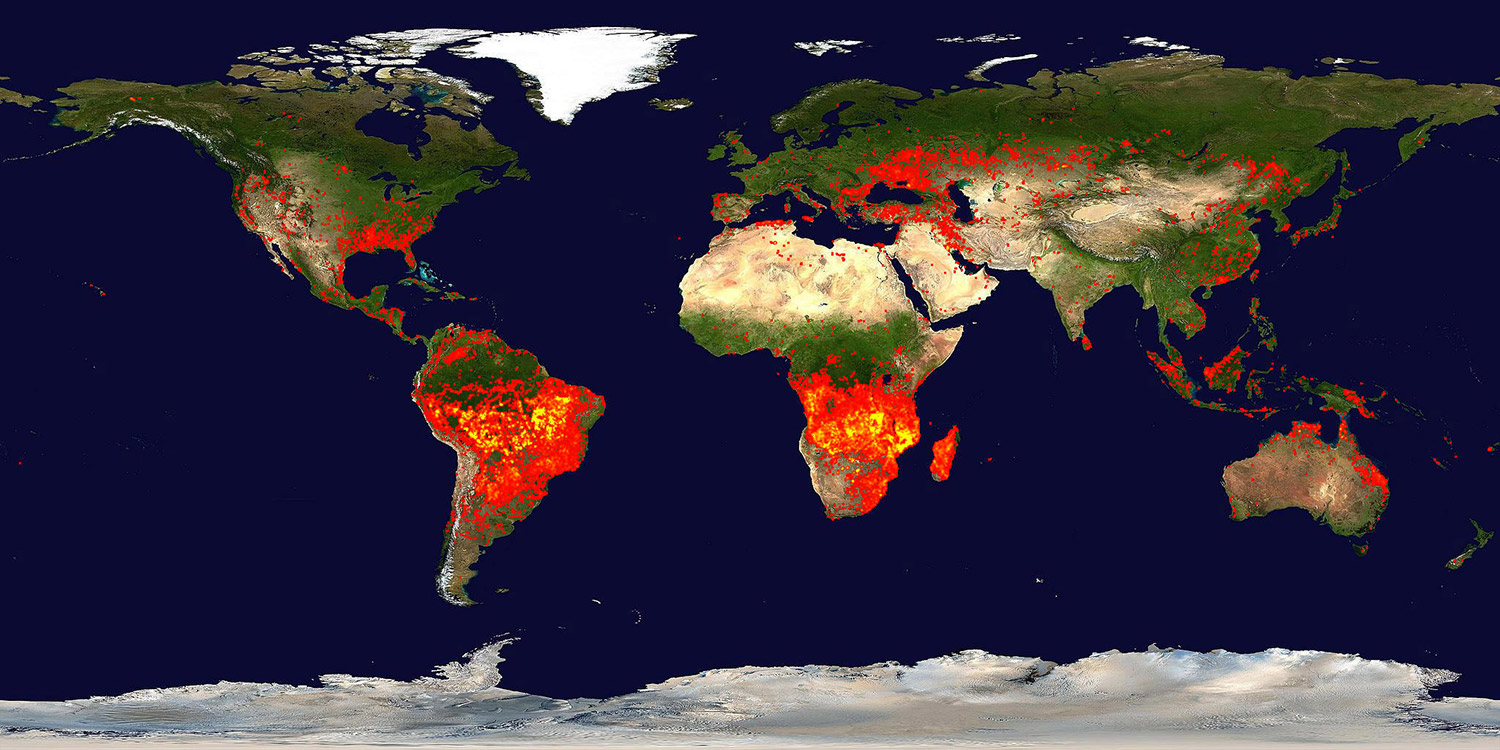
World on fire, particularly in the tropics, in the season after a super El Niño. Each red pixel is a wildfire large enough to register on NASA’s ozone-mapping satellite TOM-EP.
Sulfur signals in the Antarctic snow have revealed the importance of overlooked atmospheric chemistry for understanding climate, past and future.
Eruptions of huge volcanoes, the disruptive weather pattern known as El Niño, and a fire season from hell each left distinctive chemical marks in layers of snow excavated near the South Pole, researchers from the University of California, San Diego and France report in the Proceedings of the National Academy of Sciences the week of August 4.
Sorting out the chemical reactions that must have led to those traces revealed a process, known but overlooked, that should be included in models of climate, both forecasts of climate to come and those built to understand Earth’s early history.
“We observed huge signals from ENSO driven changes like extreme dry weather and ensuing biomass burning, which surprised me,” said Robina Shaheen, a project scientist in chemistry at UC San Diego and lead author of the report. “The pattern we saw fits signals that have been observed in pre-Cambrian rocks, which prompted us to take another look at which molecules play a role in this chemistry.”

Robina Shaheen measured sulfur isotopes captured in Antarctic snow. Credit: Susan Brown
The element sulfur is everywhere and occurs in four stable forms, or isotopes, each with a slightly different mass. Ordinary reactions incorporate sulfur isotopes into molecules according to mass.
But sometimes sulfur divvies up differently so that the relative ratios of the different isotopes is anomalous. Shaheen and her colleagues measured the direction and degree of that anomaly for individual layers of snow representing a single season’s snowfall.
The snow record they analyzed spans the years 1984 through 2001. Two large volcanoes erupted in that time including Pinatubo and Cerro Hudson, both in 1991.
Volcanoes this large inject sulfate into the stratosphere, Earth’s high atmosphere above where clouds and weather normally prevail. This sulfur shows up in the snow, lots of it, and the isotope ratio within it is anomalous.

Air heated by large fires wafts smoke into the statosphere as pyrocumulonimbus clouds. Credit: Cloud Appreciation Society
Experiments have shown this pattern can result from the influence of shortwave UV light, which doesn’t penetrate very far into the atmosphere. Sulfur anomalies in the present-day atmosphere are a sign of reactions that occurred in the stratosphere, above 25 kilometers or 82,000 feet. That is, above Earth’s shield of ozone.
Shaheen and colleagues also saw sulfur anomalies in snow that fell during years of the cyclical weather pattern called El Niño-Southern Oscillation, or ENSO. That snow held no extra sulfur, only an anomalous pattern of isotopes in the sulfate molecules. Because the pattern resembles anomalies originating from volcanic eruptions, it likely results from similar processes: photochemistry of sulfur compounds in the presence of short wave UV light, above the ozone.
Stratospheric sulfur has climate consequences we don’t yet fully understand, but it scatters incoming solar radiation. Weather cooled in the years following the eruptions of Pinatubo and Cerro Hudson. And a recent study has attributed the apparent hiatus in global warming over the past 15 years to the extreme ENSO of 1997-1998.
The surprise in the snow was for the year following this super ENSO, which had the largest sulfur anomaly of all. There was also an odd spike of potassium, which proved a valuable clue. No large volcanoes erupted, but in the dry season following record monsoons, large swaths of land burned.
“Earth was on fire,” said Mark Thiemens, professor of chemistry at UC San Diego and co-author of the report. “The Amazon, central Africa, Australia, much of Indonesia, the whole middle of the planet, the tropics, heated.”
Large fires pump smoke all the way into the stratosphere in huge pyrocumulonimbus clouds. It’s a mix of molecules like potassium, which marks forest fires in ice cores going back to the 18th century, nitrogen compounds, and sulfur in two important forms.
One is sulfur dioxide, the gas most often associated with volcanoes. The other is carbonyl sulfide, a very stable molecule that dissociates when it encounters UV light to form another source of sulfur dioxide. That extra source is the boost, they think, that produced such a large sulfur anomaly signal in the year after the super ENSO.
The role of this reaction in creating sulfur anomalies has been little considered in models of climate. That’s important because carbonyl sulfide is created whenever biomass or fossil fuels combust at high temperatures. And we’re combusting a lot of fossil fuel.
It could also be important for interpreting Earth’s history. Undisturbed snow, or snow compacted into ice, holds a record of Earth’s atmosphere, but so does stone. The oldest rocks, those older than 2.4 billion years, have sulfur anomalies as well. They are thought to have formed in an environment with little oxygen so that Earth’s lower atmosphere without its shroud of protective ozone was bathed in short wave UV light.
Carbonyl sulfide has been found in geothermal waters, volcanoes, the atmosphere of Venus, comets and dense molecular clouds in interstellar space, so it’s plausible to think it was abundant on early Earth as well. What’s interesting is that the present-day reaction, in an atmosphere of abundant oxygen, yields a sulfur isotope signal that so closely matches those found in ancient rocks.
Co-authors of the paper include Mariana Abaunza, Teresa Jackson, both members of Thiemen’s research group at UC San Diego, and Justin McCabe, a former member now a teacher at the Pacific Ridge School in Carlsbad, Calif., and Joël Savarino, of Laboratoire de Glaciologie et Géophysique de l’Environment in Grenoble, France.
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



