Studies Reveal How Cells Distinguish Between Disease-Causing and Innocuous Invaders
By:
- Kim McDonald
Published Date
By:
- Kim McDonald
Share This:
Article Content
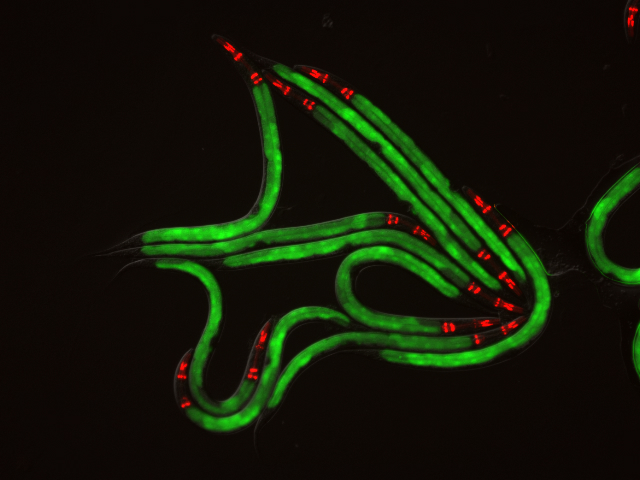
The specific mechanisms by which humans and other animals are able to discriminate between disease-causing microbes and innocuous ones in order to rapidly respond to infections have long been a mystery to scientists. But a study conducted on roundworms by biologists at UC San Diego has uncovered some important clues to finally answering that question.
In a paper published in this week’s early online issue of the journal Cell Host & Microbe, the researchers discovered that intestinal cells in the roundworm C. elegans, which are similar in structure to those in humans, internalize bacterial toxins that inactivate several host processes. This then triggers an immune response, which results in the body mounting an immediate attack against the disease-causing microbes.
“The human intestine is teeming with trillions of bacteria, most of which are innocuous, or even beneficial,” said Emily Troemel, an assistant professor of biology at UC San Diego who headed the study. “However, sometimes microbes cause disease, such as occurs in food poisoning.”
The UC San Diego study and two others published this week in the journals Cell and Cell Host & Microbe by research teams headed by Frederick Ausubel and Gary Ruvkun at the Massachusetts General Hospital and the Harvard Medical School, show that the way animal cells detect an attack by poisons or disease-causing bacteria is by monitoring the function of their own cells. If those cells detect a deficit in functions, the scientists discovered, they then trigger a variety of antibacterial or antitoxin responses against the invaders.
The roundworms proved to be the ideal laboratory model for these studies. Not only do they have intestinal cells that are similar in structure to human intestinal cells, but they are transparent and easy to maintain and study in lab.
“C. elegans provides a wonderful system in which to study questions of how humans and other animals defend themselves against attacks from disease-causing organisms,” said Troemel. “It lacks an adaptive immune system and, instead, relies solely on the evolutionarily ancient innate immune system to fight off attacks. Our findings in these roundworms may have uncovered a new ‘pathogen-specific’ branch of the innate immune system, which could function in humans as well.”
Troemel’s team of researchers—who included Tiffany Dunbar, Zhi Yan, Keir Balla and Margery Smelkinson—found in their experiments that a particular genetic system—the “ZIP-2 surveillance pathway”—was used by the roundworm in detecting an infection by the disease-causing bacterium Pseudomonas aeruginosa. The biologists also found that a specific toxin in the bacterium—“Exotoxin A”—blocks protein synthesis in the worm’s intestine.
“Surprisingly, this block leads to increased protein levels of the ZIP-2 transcription factor to ultimately induce expression of defense genes,” the scientists conclude in their paper. “Thus, a common form of pathogen attack acts to switch on host defense, allowing discrimination of pathogens from innocuous microbes.”
“In addition to P. aeruginosa Exotoxin A,” said Troemel, “there are several other bacterial toxins known to block protein synthesis, such as Diphtheria toxin, Ricin toxin and Shiga toxin. These toxins cause substantial impact on public health. For example, a recent epidemic outbreak of Shiga-toxin producing E. coli caused over 3000 cases of food poisoning in Germany leading to 39 deaths. Like Exotoxin A, these toxins can be internalized into the host cell to block protein synthesis. Perhaps the human intestine also monitors disruption of host protein synthesis to detect food poisoning, and induce a response similar to what is found in the C. elegans intestine.”
Troemel noted that it makes sense why animals have evolved systems that respond to core cellular dysfunction, rather than directly to specific toxins.
“We live in an environment filled with a wide variety of disease-causing organisms that can attack us using toxins,” she said. “While these toxins are diverse in structure, the manner by which they disrupt our cellular machinery can be very similar. Directly monitoring the functioning of our cellular machinery may provide the optimal system for early detection and response to unknown toxins or pathogens.”
The UC San Diego study was funded by the NIAID, Moores Cancer Center, Searle Scholars Program, Ray Thomas Edwards Foundation and David & Lucille Packard Foundation.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.