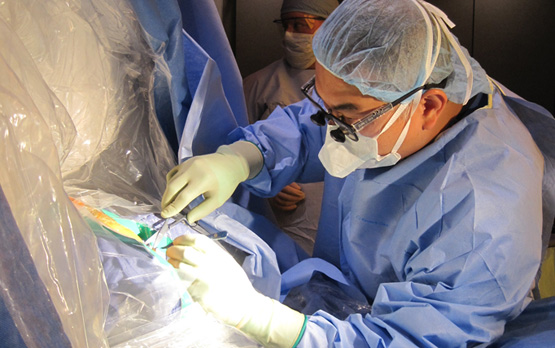
Southern California’s First Real-Time MRI-Guided Gene Therapy for Brain Cancer
Clinical trial advances Toca 511 gene therapy with precise drug delivery
By:
- Jackie Carr
Published Date
By:
- Jackie Carr
Share This:
Article Content
Neurosurgeons at the University of California, San Diego School of Medicine and UC San Diego Moores Cancer Center are among the first in the world to utilize real-time magnetic resonance imaging (MRI) guidance for delivery of gene therapy as a potential treatment for brain tumors. Using MRI navigational technology, neurosurgeons can inject Toca 511 (vocimagene amiretrorepvec), a novel investigational gene therapy, directly into a brain malignancy. This new approach offers a precise way to deliver a therapeutic virus designed to make the tumor susceptible to cancer-killing drugs.
“With chemotherapy, just about every human cell is exposed to the drug’s potential side-effects. By using the direct injection approach, we believe we can limit the presence of the active drug to just the brain tumor and nowhere else in the body,” said Clark Chen, MD, PhD, chief of stereotactic and radiosurgery and vice-chairman of neurosurgery at UC San Diego Health System. “With MRI, we can see the tumor light up in real time during drug infusion. The rest of the brain remains unaffected so the risk of the procedure is minimized.”
Toca 511 is a retrovirus engineered to selectively replicate in cancer cells, such as glioblastomas. Toca 511 produces an enzyme that converts an anti-fungal drug, flucytosine (5-FC), into the anti-cancer drug 5-fluorouracil (5-FU). After the injection of Toca 511, the patients are treated with an investigational extended-release oral formulation of 5-FC called Toca FC. Cancer cell killing takes place when 5-FC comes into contact with cells infected with Toca 511.
“Inevitably, almost all glioblastoma patients fail currently available therapy. The challenge, in part, is knowing if current drugs are actually penetrating the tumor. This MRI-guided approach will help us deliver this drug into the tumor directly to see if the drug is working,” said Santosh Kesari, MD, PhD, principal investigator and director of neuro-oncology at Moores Cancer Center. “This approach may lead to new treatment options for patients battling several other types of brain cancers.”
Previous efforts using gene therapy to treat brain cancer were largely limited by the inability to deliver the drug into the brain. Under normal conditions, the brain is protected by the blood-brain barrier but this natural defense mechanism also prevents drugs from reaching the cancer cells in patients with brain tumors. Fortunately, 5-FC crosses the blood-brain barrier, and direct injection of Toca 511 into the tumor provides a means to selectively generate chemotherapy within the tumor mass.
To ensure that the adequate amount of Toca 511 is delivered to the region of the tumor, neurosurgeons at UC San Diego Health System utilize state-of-the art MRI guidance, called ClearPoint, to monitor the delivery and injection processes in real time. The MRI-guided process provides visual confirmation that the desired amount of drug is delivered into the tumor and provides physicians the ability to make adjustments to optimize the location of drug delivery.
Participants in this clinical trial must be 18 years or older; have a single, recurrent Grade 3 or 4 glioma; and have had prior surgery, radiation, and chemotherapy. The MRI-based procedure is minimally invasive and all participants of the study were discharged from the hospital one day after surgery and resumed their normal daily activity.
The Phase 1 trial is evaluating the safety and tolerability of Toca 511 in combination with Toca FC (5-FC, extended-release tablets), and is being developed by San Diego-based Tocagen Inc.
For more information about this clinical trial at Moores Cancer Center, please call Brad Brown at 858-822-5377 or email bdbrown@ucsd.edu
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.