SDSC’s ‘Comet’ Aids in Discovery of New Organometallic Compounds
New Catalytic Process in Chemicals More Eco-Friendly & Energy-Efficient
Published Date
By:
- Jan Zverina
Share This:
Article Content
Selective oxidation plays a key role in the production of compounds widely used throughout the chemical industry. Now, according to a new study using advanced computational resources including the Comet supercomputer at the San Diego Supercomputer Center at UC San Diego, these materials and other compounds, such as those used to make polyester resins, could undergo a new catalysis process that uses less energy and generates fewer by-products than current methods.
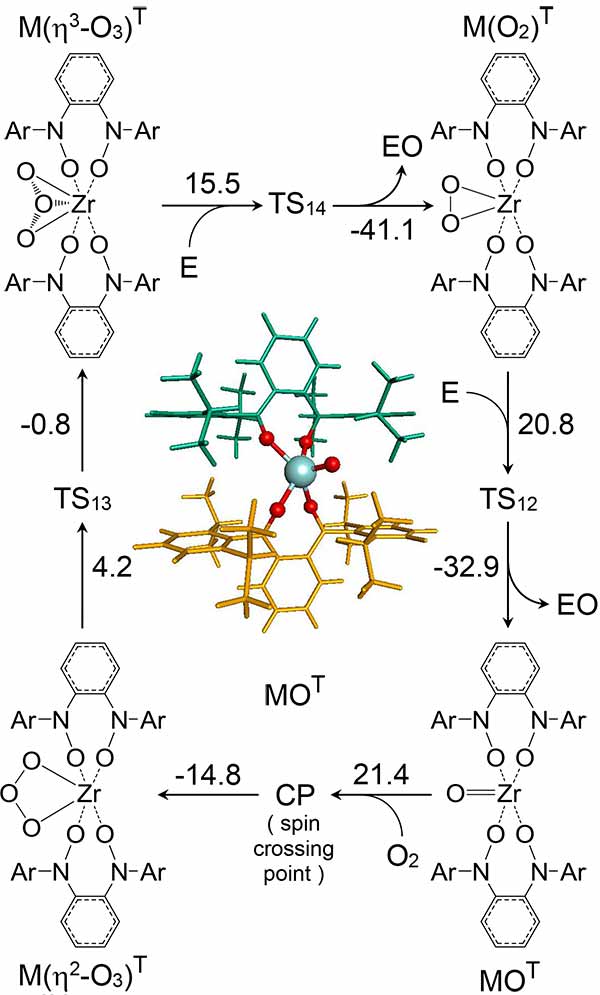
New catalyst shown in center with a zirconium (Zr) atom colored cyan, oxygen atoms (red), and organic ligands (green and orange.) Surrounding this is a computed reaction cycle for selective oxidation of ethylene to ethylene oxide over this catalyst. The number beside each arrow is the computed energy change in kilocalories per mole. Image by Thomas Manz and Bo Yang, NMSU.
The findings, recently published online in Theoretical Chemistry Accounts by Thomas Manz, a chemical engineering professor at New Mexico State University (NMSU), are significant because they can be applied to chemical compounds used widely throughout commercial industry. Manz, with his Ph.D. student, Bo Yang, used more than one million computational hours over a period of several years to produce a new class of selective oxidation catalysts.
“Molecular oxygen is the ideal oxidant, because it is present in air and can potentially produce selective oxidation without co-products,” said Manz, who works in NMSU’s Department of Chemical and Materials Engineering. “While some selective oxidation processes efficiently utilize molecular oxygen, other selective oxidation products still lack an efficient catalyst to produce them using molecular oxygen as the oxidant without co-reductant.”
Specifically, the study suggests that a novel solution-phase bidentate zirconium complex can efficiently mediate selective oxidation reactions directly with molecular oxygen, without the need for coreactants. While the Theoretical Chemistry Accounts paper focuses on ethylene epoxidation, Manz and Yang’s most recent computations, to be published in a follow-up study, show that their new catalyst can also be used in a two-catalyst process to selectively oxidize propene to propylene oxide using molecular oxygen as the oxidant without co-reductant.
“These latest findings are significant because it is much more difficult to produce propylene oxide than ethylene oxide,” according to Manz. Propylene oxide, which is used to make polyurethanes, polyester resins, and other compounds, is one of the world’s major chemical products, with global production estimated at about 15 billion pounds per year.
“We believe this is one of the first examples to date of designing a brand new catalytic route and catalyst class from scratch using quantum chemistry calculations, and one that will reduce unwanted co-products that can be environmentally unfriendly,” said Manz. “We also are convinced that this new route can result in substantial energy savings when compared with existing processes which use a co-reductant or require an oxidant other than molecular oxygen.”
Density Functional Theory calculations used by Manz and Yang to design the new catalysts and selective oxidation processes involved the use of Comet, and earlier, the Trestles system at SDSC, as well as the Stampede system at the Texas Advanced Computing Center (TACC) and the Steele compute cluster housed at Purdue University. This research was conducted via allocations on those systems via the National Science Foundation’s XSEDE (eXtreme Science and Engineering Discovery Environment) program, one of the most advanced collections of integrated digital resources and services in the world.
“The advanced state of computational power is what paved the way to discovering this new class of organometallic compounds that catalyze selective oxidation reactions by passing through eta3-ozone intermediates,” said Manz. “We thank SDSC and XSEDE’s technical support staff for their assistance, and are currently developing collaborations with experimental groups to synthesize and test these new catalysts and selective oxidation processes.”
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



