Scientists Present New Cold Facts about Antifreeze Proteins
UC San Diego chemists apply novel water model to determine the mechanisms of how antifreeze proteins attach to ice
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
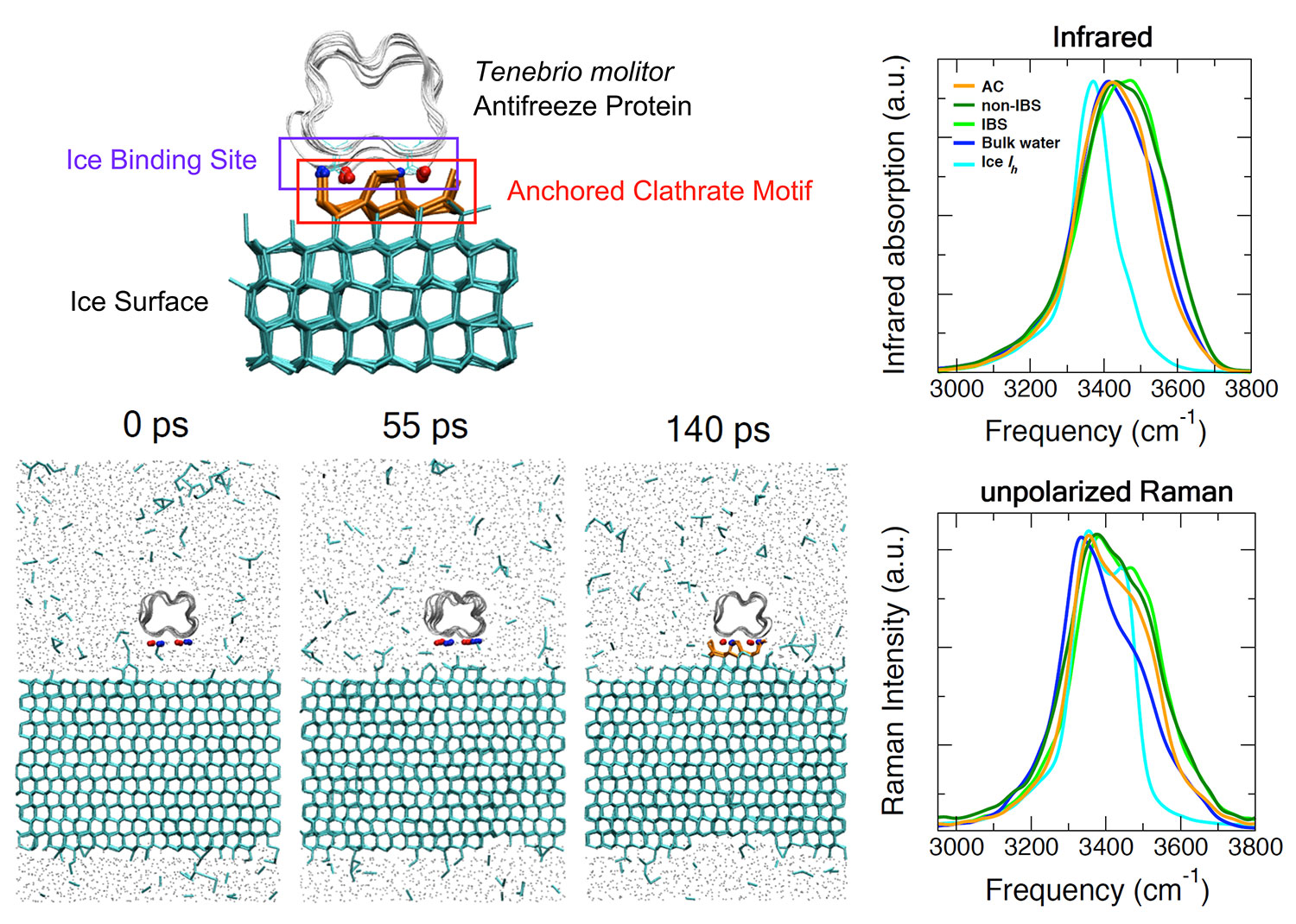
Diagram of antifreeze protein used in the study as well as the process of the binding event to the ice surface observed through simulation. Image courtesy of Daniel Moberg and Francesco Paesani
Many insects and animals have special proteins that act like car antifreeze to prevent ice from forming and spreading in their bodies amidst harsh winter temperatures. Scientists know about these antifreeze proteins (AFPs), but not so much about the mechanisms that make them work. Chemistry researchers at the University of California San Diego and the University of Utah, however, share new information about AFP function in their July 9 article published in the Proceedings of the National Academy of Sciences (PNAS). Their research results could impact a variety of industrial and natural processes, including cloud formation, as well as future scientific studies.
Scientists, like UC San Diego’s Francesco Paesani and Daniel Moberg, know that AFPs prevent water from freezing by surrounding and binding to small ice crystals, formed by water that quickly organizes into a cage-like form of ice—chemically referred to as a clathrate structure. Left unattended, these organizing crystals act as seeds and continue spreading to neighboring water molecules. The current hypothesis for how AFPs stop this from occurring focuses on the pre-ordering of an ice-like layer of water near the site of the protein that binds to the ice surface. But because it is difficult to isolate this small region from the surrounding ice and water in experiments, it has been hard to prove.
Focusing on the AFP of the mealworm beetle, (Tenebrio molitor, TmAFP), Paesani and Moberg collaborated with University of Utah Professor Valeria Molinero and her graduate student Arpa Hudait to test the hypothesis by combining state-of-the-art theoretical methods at different scales in space and time. Applying the expertise of Molinero, who specializes in simulating ice at larger scales, to a system with TmAFP in water approaching an ice surface, two observations were made: 1) the protein slowly tumbled above the ice surface and 2) TmAFP could latch-on or bind to the ice when parallel to the surface. Importantly, Molinero and Hudait discovered that this latching did not require prior organizing, or preordering, of the water into an ice-like structure.
“In insects like the mealworm beetle, the binding of many AFPs to developing ice crystals prevents further ice nucleation in their bodies,” said Hudait.
In order to isolate the watery region bound for ice and create associated spectroscopic predictions—which help scientists better understand the chemical composition of materials—the UC San Diego Department of Chemistry and Biochemistry researchers applied their highly accurate water model (MB-pol) to the research. As a result, the team of scientists were able to model, for the first time, both infrared and Raman spectra of water at the interface with TmAFP through computer simulations.
“Simulations have the advantage of being able to isolate any desired region, though this is only useful if the underlying theory is accurate enough,” explained Paesani.
Moberg added that the results show that infrared spectroscopy is unlikely to provide much information on the anchored clathrate structure. “Raman spectroscopy, however, should show differences if experimentalists can isolate the signal from the binding site or anchored clathrate.”
According to Molinero, besides providing insight on how AFPs function, the study’s findings can be applied to ice nucleating proteins in the atmosphere. “We predict that preordering could emerge on the large surfaces of aggregated ice-nucleating proteins, where it may serve the opposite role of assisting with ice nucleation in clouds.”
This research was supported by the National Science Foundation (award CHE-1305427 “Center for Aerosol Impacts on Chemistry of the Environment”) and the Center for High Performance Computing at the University of Utah (technical support and computer time).
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



