Scientists at UC San Diego Introduce a Battery for Extreme Temperatures
A new electrolyte design may have applications in mining, space exploration and more
Published Date
Story by:
Media contact:
Share This:
Article Content
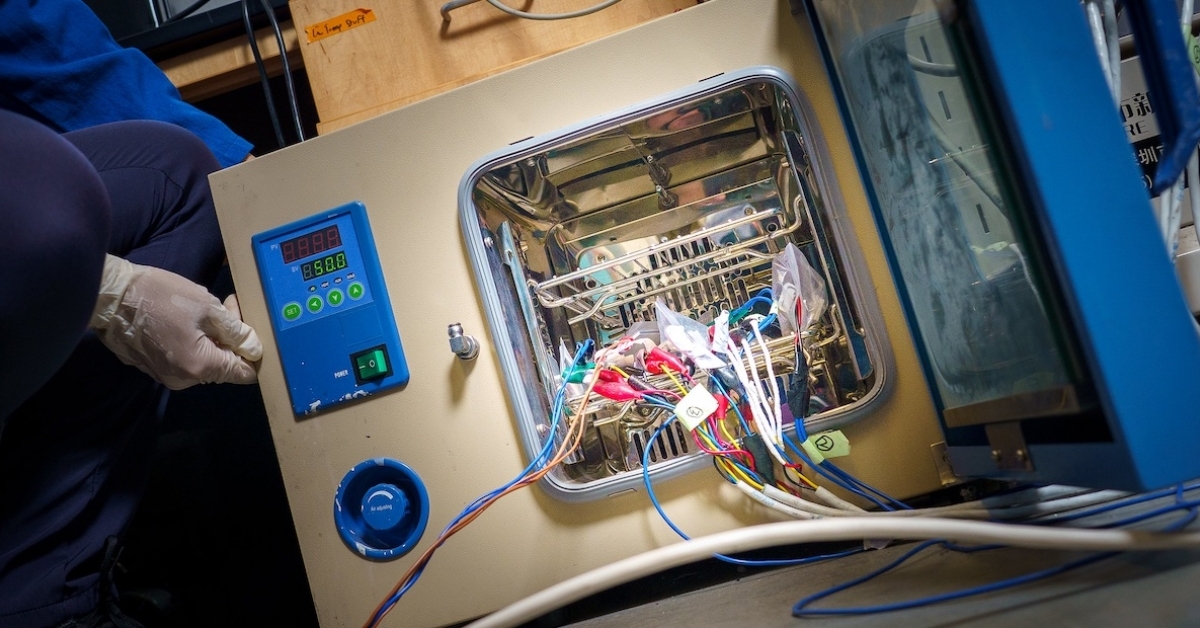
A team of nanoengineers from UC San Diego has published groundbreaking research on batteries built to deliver high-power output under the hottest and coldest conditions. This work provides insights into the electrolyte design that may overcome the operational limits of batteries in extreme temperatures and weather conditions.
“It basically enables your batteries to be used in any state in the United States as well as in deep sea or space,” said Zheng Chen, a professor at the Jacobs School of Engineering at UC San Diego, affiliated with the UC San Diego Sustainable Power and Energy Center at and coauthor of the paper. “You won’t need to worry about cells catching fire easily in Arizona or freezing in Wisconsin.”
The work was performed by a research team combining Chen’s group and a group led by Y. Shirley Meng, a professor of molecular engineering at the University of Chicago and adjunct professor of nanoengineering at UC San Diego. It’s explored in depth in a paper published in the Oct. 25, 2022 edition of Advanced Materials.
The potential applications for such technology are numerous. “NASA’s space exploration missions could be the ideal applications for these batteries,” said Yijie Yin, a nanoengineering PhD student in the Materials Science and Engineering program at UC San Diego and coauthor of the paper. “Some military functions or mining companies are likely to use these sorts of batteries to power their detection equipment under the deep ocean.”
Such extreme environments require special batteries with long storage and operation life as well as high energy and power density. The most promising such batteries, those using lithium fluorinated-carbon, or Li/CFx, generally have significant limitations due to their sluggish bulk electrolyte transport and increased charge transfer impedance, which can cause poor performance under low-temperature conditions.
These new batteries perform well in extremely high and low temperatures thanks to a novel electrolyte made from a liquid solution of dibutyl ether mixed with a lithium salt. With a boiling point of 141 °C, dibutyl stays liquid in scorching heat. Also, dibutyl ether molecules bind weakly to lithium ions, and this weak molecular interaction improves extreme-temperature performance. This weak molecular interaction, the researchers had discovered in a previous study, improves battery performance at sub-zero temperatures.
Using systematic X-ray photoelectron spectroscopy integrated with transmission electron microscopy characterizations, the team evaluated the interface of CFx for low-temperature performance. The fast transport and anion-pairing solvation structure of the electrolyte reduced charge-transfer resistance at low temperatures, enhancing performance of Li/CFx cells (1690 Wh kg−1, −60 °C based on active materials). Using 50 mg cm−2 loading electrodes, the Li/CFx still displays 1530 Wh kg−1 at −60 °C.
“When we first proposed the electrolyte formulations and found a significantly improved performance, we couldn’t locate the dominating factor,” said Yin. “We carefully designed the experiments, factored out each parameter and finally attributed the improved performance mainly to the bulk physical and electrochemical properties of the electrolytes.”
Following publication, the team plans to continue and broaden its work. “We realized there is limited understanding of the temperature effect of the electrolyte’s solvation structure,” said Yin. “The investigation of how the solvation structure evolves with the decrease of the temperature is scientifically interesting and will guide the future development of low-temperature batteries.”
Paper: “Fast-transport and Anion-pairing Liquefied Gas Electrolytes.”
Coauthors include Yijie Yin, Materials Science and Engineering Program, UC San Diego; Alex Liu,, Baharak Sayahpour, Ganesh Raghavendran, Guorui Cai, Bing Han and Matthew Mayer, Department of Nanoengineering, UC San Diego; Noah B. Schorr, Department of Power Sources R&D, Sandia National Laboratories; Timothy N. Lambert, Department of Photovoltaics and Materials Technology, Sandia National Laboratories; and Katharine L. Harrison, Nanoscale Sciences Department, Sandia National Laboratories. Corresponding authors include Weikang Li, Materials Science and Engineering Program, UC San Diego; Zheng Chen, Materials Science and Engineering Program, Department of Nanoengineering and Sustainable Power and Energy Center, UC San Diego; and Y. Shirley Meng, Materials Science and Engineering Program, Department of Nanoengineering and Sustainable Power and Energy Center, UC San Diego; and Pritzker School of Molecular Engineering, University of Chicago.
This work was supported partially by the Laboratory Directed Research and Development program (Project 218253) at Sandia National Laboratories, a multi-mission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC., a wholly owned subsidiary of Honeywell International, Inc., for the U.S. Department of Energy's National Nuclear Security Administration under contract DE-NA-0003525.
Share This:
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.