Researchers Develop a Remote-Controlled Cancer Immunotherapy System
Published Date
By:
- Liezel Labios
Share This:
Article Content
A team of researchers has developed an ultrasound-based system that can non-invasively and remotely control genetic processes in live immune T cells so that they recognize and kill cancer cells.
There is a critical need to non-invasively and remotely manipulate cells at a distance, particularly for translational applications in animals and humans, researchers said.
The team developed an innovative approach to use mechanogenetics—a field of science that focuses on how physical forces and changes in the mechanical properties of cells and tissues influence gene expression—for the remote control of gene and cell activations. Researchers used ultrasound to mechanically perturb T cells, and then converted the mechanical signals into genetic control of cells.
A schematic drawing of ultrasound-induced cell activation and gene expression.
In this study, researchers show how their remote-controlled mechanogenetics system can be used to engineer chimeric antigen receptor (CAR)-expressing T cells that can target and kill cancer cells. The engineered CAR-T cells have mechano-sensors and genetic transducing modules that can be remotely activated by ultrasound via microbubble amplification.
“CAR-T cell therapy is becoming a paradigm-shifting therapeutic approach for cancer treatment,” said bioengineering professor Peter Yingxiao Wang at the University of California San Diego. “However, major challenges remain before CAR-based immunotherapy can become widely adopted. For instance, the non-specific targeting of CAR-T cells against nonmalignant tissues can be life-threatening. This work could ultimately lead to an unprecedented precision and efficiency in CAR-T cell immunotherapy against solid tumors, while minimizing off-tumor toxicities.”
The team brings together the laboratories of professors Wang and Shu Chien, both bioengineering professors at the Jacobs School of Engineering and the Institute of Engineering in Medicine at UC San Diego, in collaboration with professors Kirk Shung of the University of Southern California and Michel Sadelain at Memorial Sloan Kettering Cancer Center in New York. Researchers present their findings in the Jan. 15 issue of the Proceedings of the National Academy of Sciences, with UC San Diego Ph.D. candidate Yijia Pan as the first author.
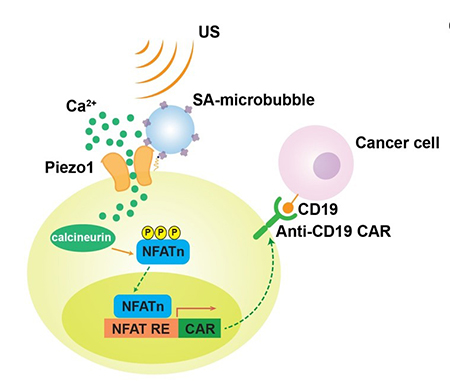
Researchers found that microbubbles conjugated to streptavidin can be coupled to the surface of a cell, where mechanosensitive Piezo1 ion channels are expressed. Upon exposure to ultrasound waves, microbubbles vibrate and mechanically stimulate Piezo1 ion channels to let calcium ions inside the cell. This triggers downstream pathways, including calcineurin activation, NFAT dephoshorylation and translocation into the nucleus. The nucleus-translocated NFAT can bind to upstream response elements of genetic transducing modules to initiate gene expression of chimeric antigen receptor (CAR) for the recognition and killing of target cancer cells.
Paper title: "Mechanogenetics for the remote and non-invasive control of cancer immunotherapy." Authors of the study are Yijia Pan, Ziliang Huang, Molly Allen, Yiqian Wu, Ya-Ju Chang, Shu Chien and Yingxiao Wang at UC San Diego; Sangpil Yoon, Changyang Lee and K. Kirk Shung at University of Southern California; and Jie Sun and Michel Sadelain at Memorial Sloan Kettering Cancer Center, New York.
This work was supported by the National Institutes of Health (grants HL121365, GM125379, CA204704 and CA209629), the National Science Foundation (grants CBET1360341 and DMS1361421) and the Beckman Laser Institute Foundation.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



