Researchers Make Gemstones with Salty, Soapy Water for the First Time
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
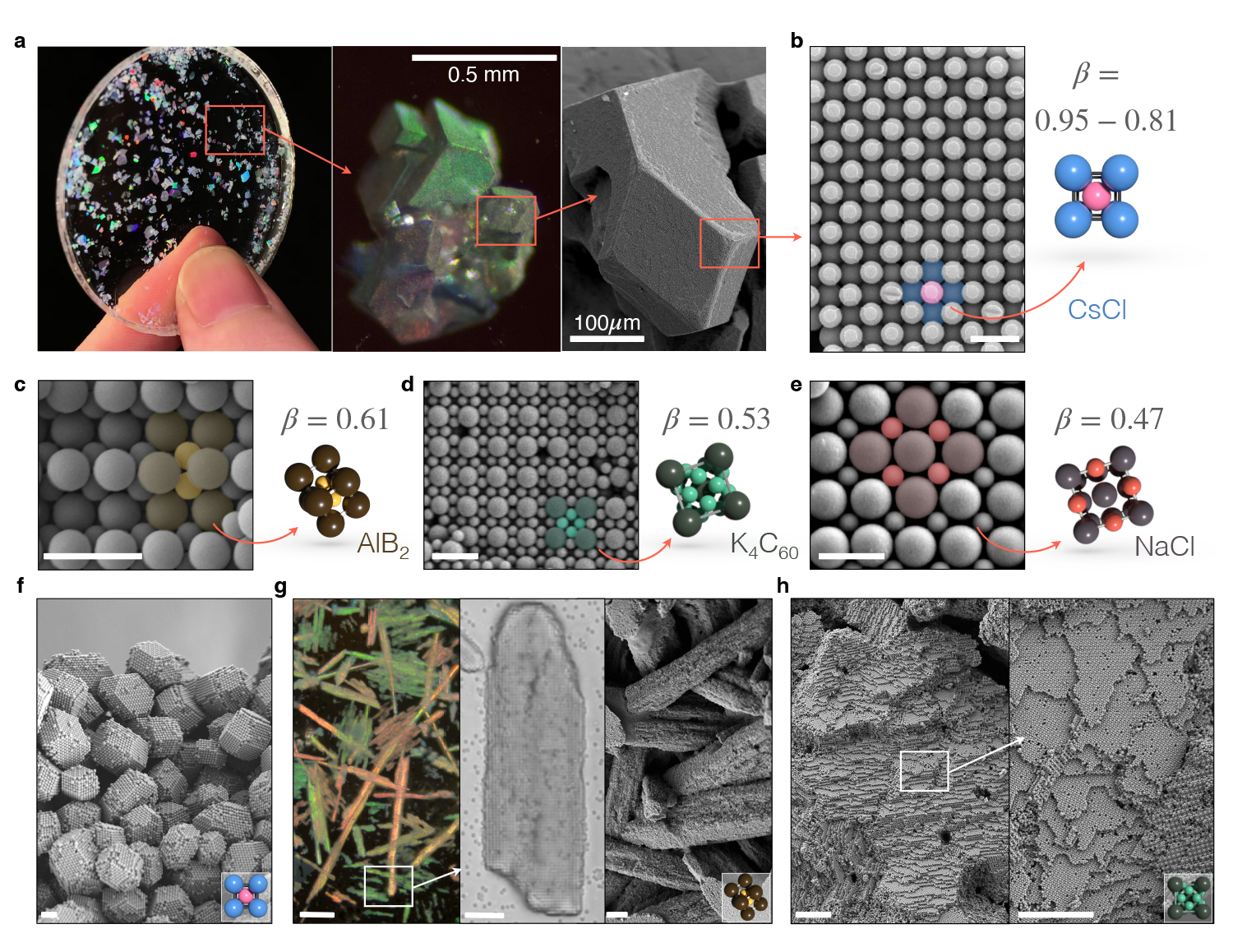
Colloidal gemstones. Fixed ionic colloidal crystals can be safely dried and manipulated. Figure shows a collection of millimeter-sized iridescent monocrystalline solids embedded in a refractive index matching epoxy resin. Scale bar 100µm. Figure courtesy of Jérémie Palacci, UC San Diego
Collaborators from the University of California San Diego and New York University (NYU) used salt, soap and water to make “bling” with a proposed novel experiment by UC San Diego’s Jérémie Palacci to form ionic colloidal crystals from common colloids. Their research findings are published in the April 22 issue of Nature.
An example of a colloidal crystal is opal, famous as an October birthstone and for its colorful brilliance. This trait, historically considered magical, comes from the gemstone’s silica nanospheres that organize into a lattice-like structure comparable to the wavelength of visible light.
Salt, on the other hand, is an ionic solid, historically worth its weight in gold. Its structure is composed of oppositely charged ions. Nature excels at building crystals from molecular species that interact electrostatically. In the popular seasoning, positively charged sodium and negatively charged chlorine assemble into the common crystals of table salt.
“Researchers aim to achieve photonic crystals, materials that could manipulate light in unusual fashion—think the invisibility allowed by a cloaking device,” said Palacci, adding that this would require the formation of large-scale crystals, like table salt, from micron-scale building blocks (micron being a unit of measurement equal to about 39 millionths of an inch; the average cross-section of a human hair is 50 microns). “Unfortunately, on the micron scale, colloids in water defy the idea of forming crystals from oppositely charged partners. Instead, they chaotically aggregate instead, lacking the degree of organization needed in crystalline materials to manipulate light.”
In the paper titled, “Ionic solids from common colloids,” Palacci, an assistant professor of physics, NYU’s Stefano Sacanna and other team researchers lay out a scenario where virtually any charged colloid can act as a model ion in water, assembling with oppositely charged partners into crystal structures that were previously only accessible via particle interactions.
“We outline how we formed ionic colloidal crystals in water through a general approach we refer to as ‘polymer attenuated Coulombic self-assembly, or PACS,’” said Sacanna, corresponding author on the study. PACS is equivalent to using soap, a non-ionic surfactant.
According to Sacanna, the key to crystallization is the team’s use of neutral polymers to keep particles separated by well-defined distances, allowing researchers to tune the attractive overlap of electrical double layers, directing particles to disperse, crystallize or become permanently fixed on demand.
“We demonstrated the nucleation and growth of macroscopic single crystals by using the amount of salt in solution as a fine-tuning knob for assembly,” explained Sacanna.
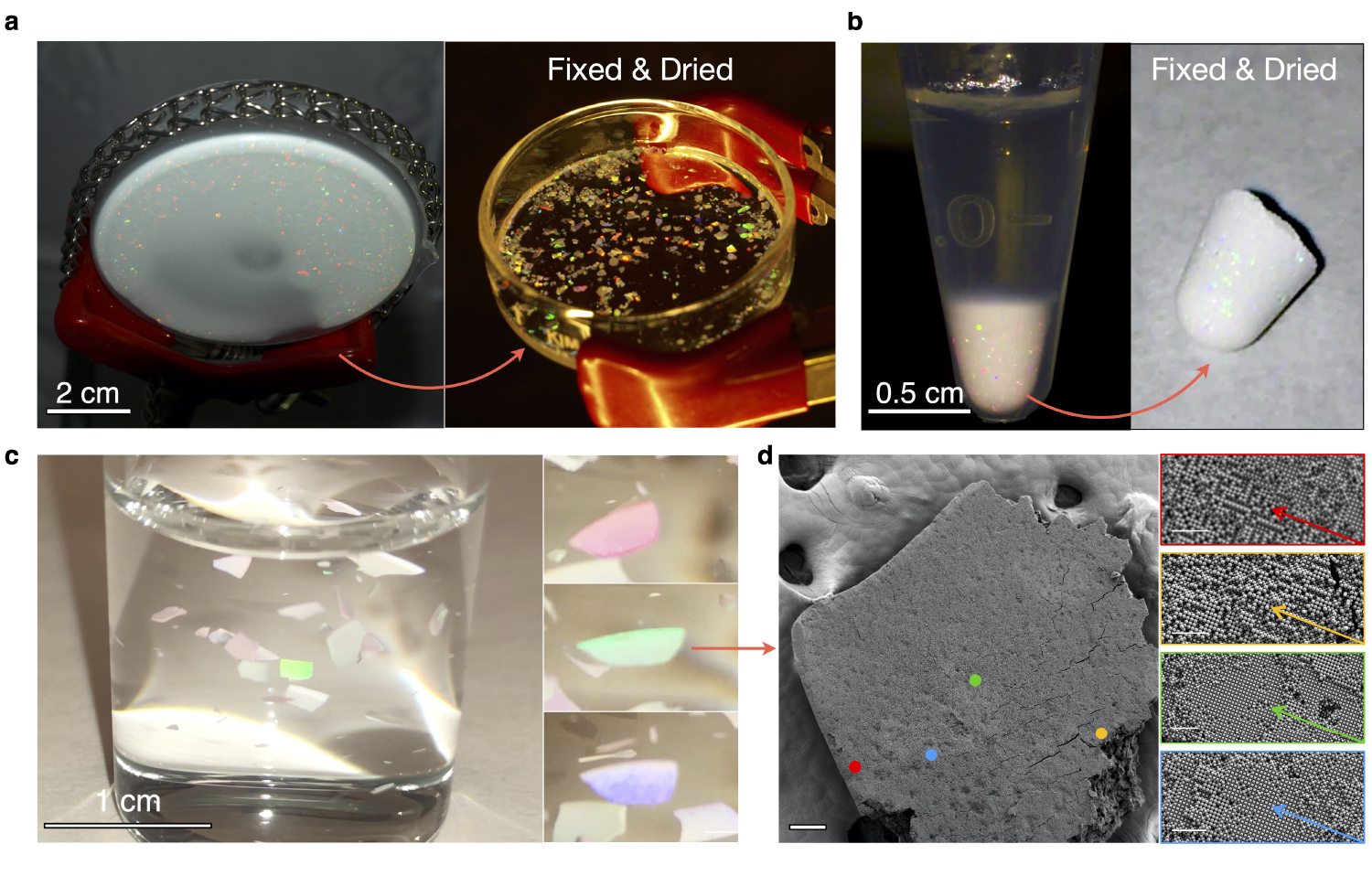
Iridescent macroscopic single crystals. Figure courtesy of Jérémie Palacci, UC San Diego
Applying a twist to the classical theory that underlies the stability of colloidal suspensions (e.g., paint or milk), the research team made a simple estimation of attractive and repulsive forces to predict assembly behavior in both simulations and experiments. Using a broad variety of colloidal particles and commercial polymers, they selected ionic colloidal crystals based on particle size ratios.
“Once fixed by simply diluting-out solution salts, crystals are pulled out of the water for further manipulation, demonstrating a faithful translation from solution-phase assembly to dried solid structures,” explained Theodore Hueckel, the NYU graduate student, who led the study. “With free-standing colloidal gemstones on display, PACS proves to be a practical route to nanofabrication by transmuting common colloidal particles into model colloidal ions.”
Sacanna explained that the delicate self-assembled architectures are then transformed into real materials via a quick and chemistry-free single-step dilution.
“Sustained by an ever-growing library of colloidal building blocks, ionic colloidal crystals hold the potential to evolve well beyond the structural complexity of the atomic solids they were inspired from,” said Sacanna. “Our assembly approach represents a concrete step forward towards a predictable and truly modular microfabrication technology whereby bulk functional materials nucleate and grow spontaneously from soups of common colloids; salt to taste.”
The researchers—Palacci, Sacanna, Hueckel and Glen Hocky, also from NYU—were supported in their work by a National Science Foundation CAREER award (DMR-1653465); the Materials Research Science and Engineering Center (MRSEC); NSF’s MRI programs (award numbers DMR-1420073 and DMR-0923251); NSF grant (no. DMR-1554724); NYU’s High Performance Computing and the Sloan Foundation (grant no. FG-2017-9392).
Share This:
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



