Researchers ‘Handed’ $4M to Boot-Up Mirror Cell Synthesis
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
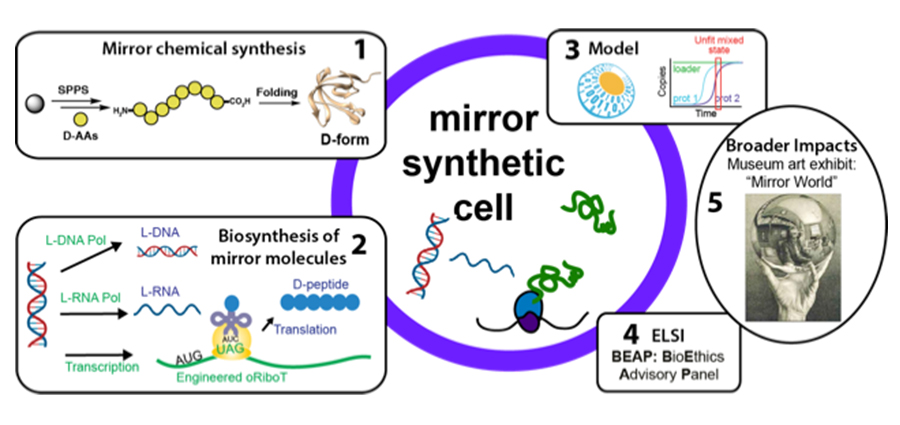
Booting up a synthetic mirror cell. The five key threads aimed at building a mirror cell. Graphic courtesy of Neal Devaraj and Farren Isaacs
An early trait parents notice in their kids is which hand they use to hold things. Scientists notice a similar trait they call handedness in some molecules. These “chiral” (from the Greek word for hand) molecules are not superimposable on their mirror images, and learning more about them could transform basic science, bioengineering and biotechnology.
With a new grant of $4 million from the National Science Foundation, UC San Diego’s Neal Devaraj and Yale University’s Farren Isaacs will work to design, construct and safely deploy mirror DNA, RNA and proteins; predict the physiology of cells with mirror components and assess the risks and rewards of mirror life.
“A hallmark of life on Earth is homochirality, or the fact that many of the key biological molecules possess the same chirality. We often refer to this property as “handedness,” as our left hand and right hands are not superimposable yet are mirror images of one another,” explained Devaraj. “Our combined synthetic, experimental and computational approach will provide the first comprehensive analysis of the physiology of chirality and why natural life evolved to one chirality.”
The 2019 Eli Lilly Award winner said that while living organisms possess a specific chirality, theoretically, an organism with biological molecules opposite chirality should be functional since handedness of molecules doesn’t impact their physical properties. So the research team will develop an integrated framework of the molecular origins of cellular phenotypes without ignoring chirality, thus leading to new modeling tools that can be used to explore natural biology.
“The analysis will also shed light on the benefits and costs of introducing new chemistry into cells,” noted Devaraj.
According to the professor of chemistry and biochemistry, the ultimate objective of the project is to develop the scientific know-how and capabilities to routinely produce mirror biological molecules, which position the team to boot-up a synthetic mirror cell. Among the project’s considerations, however, are the ethical, legal, environmental and social implications around building such a cell.
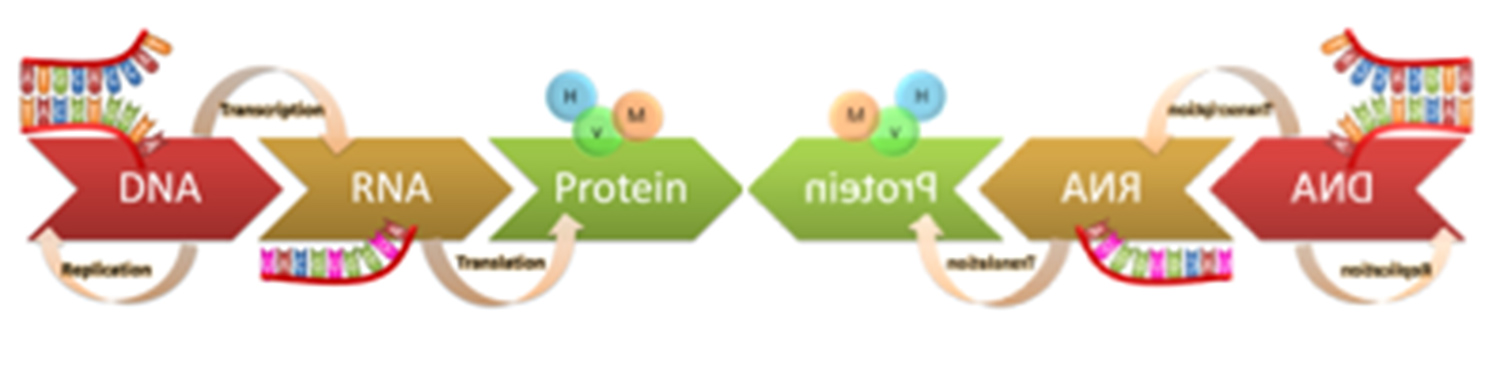
Mirror central dogma. The central dogma of molecular biology uses homochiral biomolecules to faithfully replicate and express the genome and sustain life. A synthetic cell that uses the reverse chiral biomolecules could establish a mirror world and set the stage for attaining a comprehensive understanding of the role of chirality in biology. Figure courtesy of Neal Devaraj and Farren Isaacs
“The bioethical considerations related to this project will identify limitations in our current understanding about how synthetic cells and mirror biology fit into the broader landscape of synthetic biology, and if the project leads to new societal or environmental concerns,” said Isaacs, associate professor in the Department of Molecular, Cellular & Developmental Biology at Yale.
To address these broader impacts, Devaraj will engage Professor of Sociology and Tata Chancellor’s Chair in Social Sciences John Evans, co-director of the Institute for Practical Ethics, who will lead a bioethics advisory panel, and Pinar Yoldas, assistant professor of Visual Arts at UC San Diego. Together, the research team will use the project to help train the next generation of interdisciplinary scientists with a new set of tools and unprecedented knowledge about designing and engineering living systems. Additionally, the researchers will illustrate chirality and the mirror biosphere with a public museum exhibition called, “The Mirror World,” at the San Diego Museum of Contemporary Art.
The ambitious basic and applied science project not only will give scientists a better understanding of the impact of chirality on biology, it also will spur the development of new technologies in bioconjugation, directed evolution and synthetic biology, with potential to expand the biosphere.
This collaborative project is funded by the National Science Foundation (award no. EF-1935372) over four years and includes the work of Irene Chen (UCLA), Jonathan Karr (Icahn School of Medicine at Mt. Sinai) and Kate Adamala (University of Minnesota).
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



