Researchers Develop an LSD Analogue with Potential for Treating Schizophrenia
UC San Diego biologist’s lab is the first to apply modern 3D electron microscopy techniques to psychedelic-treated brain tissue
Story by:
Published Date
Article Content
Researchers at University of California campuses at Davis and San Diego have developed a new, neuroplasticity-promoting drug closely related to LSD that harnesses the psychedelic’s therapeutic power with reduced hallucinogenic potential.
The research, published in Proceedings of the National Academy of Sciences and coauthored by UC San Diego School of Biological Sciences Assistant Professor Uri Manor, highlights the new drug’s potential as a safer and more effective treatment option for conditions like schizophrenia, where psychedelics are not prescribed for safety reasons. The compound also may be useful for treating other neuropsychiatric and neurodegenerative diseases characterized by synaptic loss and brain atrophy. The study was supported by the National Institutes of Health.
To design the drug, dubbed JRT, researchers flipped the position of just two atoms in LSD’s molecular structure. The chemical flip reduced JRT’s hallucinogenic potential while maintaining its neurotherapeutic properties, including its ability to spur neuronal growth and repair damaged neuronal connections that are often observed in the brains of those with neuropsychiatric and neurodegenerative diseases.
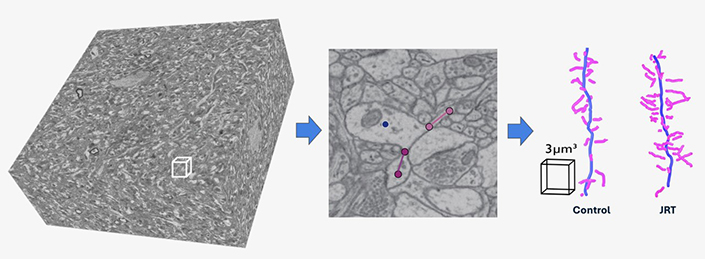
Using high-resolution 3D electron microscopy, the Manor Lab reconstructed brain tissue from the prefrontal cortex of mice treated with JRT. The analysis revealed a dramatic increase in dendritic spine density — key structures for neural connectivity — compared to untreated controls. This visualization underscores the ability of non-hallucinogenic psychoplastogens like JRT to promote robust synaptogenesis, providing an unprecedented view of their effects at the ultrastructural level.
“Basically, what we did here is a tire rotation,” said corresponding author David E. Olson, director of the Institute for Psychedelics and Neurotherapeutics and a professor of chemistry, and biochemistry and molecular medicine at UC Davis. “By just transposing two atoms in LSD, we significantly improved JRT’s selectivity profile and reduced its hallucinogenic potential.”
JRT exhibited powerful neuroplastic effects and improved measures in mice relevant to the negative and cognitive symptoms of schizophrenia, without exacerbating behaviors and gene expression associated with psychosis.
“No one really wants to give a hallucinogenic molecule like LSD to a patient with schizophrenia,” said Olson, who is also co-founder and chief innovation officer of Delix Therapeutics, a company that aims to bring neuroplastogens to the market. “The development of JRT emphasizes that we can use psychedelics like LSD as starting points to make better medicines — medicines that can be used in patient populations where psychedelic use is precluded.”
“We are incredibly excited about the therapeutic potential of non-hallucinogenic psychedelic analogs like JRT,” said Manor, who also serves as faculty director of UC San Diego’s Goeddel Family Technology Sandbox. “This work is a perfect example of how cutting-edge chemistry, neuroscience and imaging can come together to push the boundaries of biomedical research.”
Manor and members of his laboratory contributed 3D electron microscopy data and analysis to the study, which provided an unprecedented look at the neuronal ultrastructural changes induced by JRT and revealed profound synaptic growth and connectivity enhancements in the brain. The study demonstrates the power of neuroplasticity-promoting compounds to drive neuroplasticity in a similar fashion as psychedelics, but without hallucinogenic effects, which could provide new treatment options for patients with neuropsychiatric and neurodegenerative diseases, he said. He proposes this imaging approach will allow researchers to distinguish subtle yet crucial differences between compounds that have nearly identical receptor-binding profiles but vastly different acute and long-term downstream effects. Such details will be critical for advancing precision medicine in this ongoing area of research.
“This study is a testament to the power of interdisciplinary collaboration — bringing together expertise in chemistry, pharmacology, neuroscience and advanced imaging to deliver insights that wouldn’t have been possible in isolation,” said Manor. “It’s an exciting moment for the field, and I am thrilled to be part of it.”
Testing JRT’s potential
Olson said that it took his team nearly five years to complete the 12-step synthesis process to produce JRT. The molecule was named after Jeremy R. Tuck, a former graduate student in Olson’s laboratory, who was the first to synthesize it and is a co-first author of the study along with Lee E. Dunlap, another former graduate student in Olson’s laboratory.
Following JRT’s successful synthesis, the researchers conducted a battery of cellular and mouse assays that demonstrated the drug’s neuroplastic effects and improved safety profile relative to LSD.
Key findings included:
- JRT and LSD have the exact same molecular weight and overall shape, but distinct pharmacological properties.
- JRT is very potent and highly selective for binding to serotonin receptors, specifically 5-HT2A receptors, the activation of which are key to promoting cortical neuron growth.
- JRT promoted neuroplasticity, or growth between cellular connections in the brain, leading to a 46% increase in dendritic spine density and an 18% increase in synapse density in the prefrontal cortex.
- JRT did not produce hallucinogenic-like behaviors that are typically seen when mice are dosed with LSD.
- JRT did not promote gene expression associated with schizophrenia. Such gene expression is typically amplified with LSD use.
- JRT produced robust anti-depressant effects, with it being around 100-fold more potent than ketamine, the state-of-the-art fast-acting anti-depressant.
- JRT promoted cognitive flexibility, successfully addressing deficits in reversal learning that are associated with schizophrenia.
“JRT has extremely high therapeutic potential. Right now, we are testing it in other disease models, improving its synthesis, and creating new analogues of JRT that might be even better,” Olson said.
A more effective treatment for schizophrenia
Olson emphasized JRT’s potential for treating the negative and cognitive symptoms of schizophrenia, as most current treatments produce limited effects on anhedonia — the inability to feel pleasure — and cognitive function. Clozapine is the one exception, but it has major side effects and is currently only used as a last resort for those severely afflicted with schizophrenia.
“Clozapine has nasty, nasty side effects, including weight gain, metabolic issues, sedation and agranulocytosis— a condition characterized by dangerously low levels of white blood cells,” Olson said. “It’s only reserved for treatment-resistant schizophrenia.”
Unlike clozapine, JRT didn’t promote such side effects. Olson hypothesized that this is due to JRT’s more selective pharmacology.
Olson and his team are currently testing JRT’s potential against other neurodegenerative and neuropsychiatric diseases.
Additional coauthors include Yara A. Khatib, Cassandra J. Hatzipantelis, Sammy Weiser Novak, Rachel M. Rahn, Alexis R. Davis, Adam Mosswood, Anna M. M. Vernier, Ethan M. Fenton, Isak K. Aarrestad, Robert J. Tombari, Samuel J. Carter, Zachary Deane, Yuning Wang, Arlo Sheridan, Monica A. Gonzalez, Arabo A. Avanes, Noel A. Powell, Milan Chytil, Sharon Engel, James C. Fettinger, Amaya R. Jenkins, William A. Carlezon Jr., Alex S. Nord, Brian D. Kangas, Kurt Rasmussen, Conor Liston and Uri Manor.
The research reported on here was funded by grants from the National Institutes of Health, the UC Davis Provost’s Undergraduate Fellowship, the Camille and Henry Dreyfus Foundation, the Dr. Mohsen Najafi Research Award in Medicinal Chemistry, the Boone Family Foundation, Hope for Depression Research Foundation, the Pritzker Neuropsychiatric Disorders Research Consortium, the L.I.F.E. Foundation, the Chan-Zuckerberg Initiative Imaging Scientist Award, and a National Science Foundation NeuroNex2 Award.
— Adapted from a UC Davis news story
Share This:
You May Also Like
UC San Diego is Strengthening U.S. Semiconductor Innovation and Workforce Development
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



