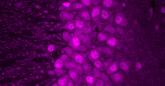
Recognizing Sex Differences in Disease Can Improve Treatments for All
Addressing these differences at the cellular level could help close the gap in health disparities in heart disease and benefit everyone.
Published Date
Story by:
Media contact:
Topics covered:
Share This:
Article Content
One-size-fits-all hats are okay, but one-size-fits-all medical treatments don’t cut it. A new study shows that drug treatment outcomes are significantly different for a type of heart valve disease, called aortic valve stenosis, based on how the disease progresses in males versus females. The research, led by bioengineers at the University of California San Diego, with collaborators at the National University of Singapore (NUS), reveals that an artificial intelligence-derived digital medicine platform can help optimize drug therapies based on cellular-scale sex differences.
The team published their results on June 6 in Science Advances.
Aortic valve stenosis, or AVS, affects nearly one in eight adults aged 75 and older. When the opening of the valve between the lower left heart chamber and the aorta narrows, the heart has to work harder to pump blood to the body. Left untreated, the disease can cause life-threatening complications, including heart failure.

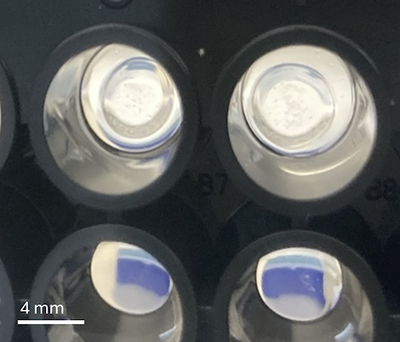
Study co-authors Brandon Vogt (left) and Megan Chavez (right) form the hydrogels to mimic the stiffness of aortic valve tissue. Photo credit: Aguado lab
For severe cases, the main treatment is valve replacement. However, replacement surgery can have complications, and not all patients are good candidates for the procedure. Ideally, the disease would be treated at the early stages with pharmacological treatments such as a family of medications called inhibitors, but there currently are no such approved options.
“As of now, most inhibitors used to stop aortic valve disease, or at least attempt to stop aortic valve disease, have not been effective in clinical trials,” said study senior author Brian Aguado, professor in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering.
Part of the challenge, he added, is that AVS is a sex-dependent disease. The opening of the aortic valve narrows due to stiffening of the valve, but the cause of that tissue stiffening is different in males and females. At the earliest stages of AVS, males develop calcium buildup, while females form fibrotic tissue.
While Aguado’s lab determined that a gene on the Y chromosome is a key driver for valve calcification in males in a prior Science Advances study, it’s clear there are multiple signaling pathways that can lead to AVS. That’s why using just one drug to treat the disease, as done in those clinical trials, isn’t enough to stop it.
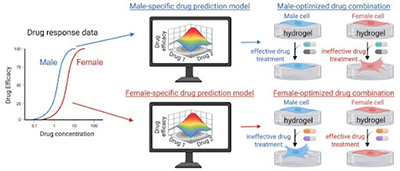
Now, in their latest study, the Aguado Lab established a framework to optimize sex-specific drug combinations to treat AVS, with help from computational tools.
The environment matters
To culture cells and screen multiple drugs on them, researchers typically use standard Petri dishes or plastic well plates. However, those surfaces are very stiff and not reflective of the environment of the valve tissue. Rather, aortic valve tissue is fairly soft similar to Jell-O, according to Aguado.
So instead the research team used biomaterials called hydrogels that they can engineer to mimic the stiffness of healthy and damaged aortic valve tissue. By culturing the tissue cells on the hydrogels, the researchers found that drugs can have significantly different effectiveness compared to culturing on plastic.
“It shows how important it is to culture the cells in the environment that they’re supposed to be in, or at least closely matches the environment that the cells are typically in,” said Aguado. “Because if you culture cells on plastic and you try to do drug screenings, you’re not really testing how cells will respond to drugs while residing in the tissue.”
“But one drawback of that is the throughput capabilities,” added co-first author Brandon Vogt, a bioengineering PhD student in Aguado’s lab.
It takes more resources and time to make multiple hydrogels and seed the cells on the hydrogels than it does to just use readily-available plastic well plates, he explained.
And the researchers wanted to screen a lot of drug combinations: different multiple-drug mixtures out of eight possible experimental inhibitors, each at three different doses, comes out to over 6,000 unique combinations. Then each one would need to be tested on biologically male and biologically female cells.
For help, the team turned to AI. Researchers from NUS, including study co-author Dean Ho, professor and director of the Institute for Digital Medicine (WisDM), NUS Yong Loo Lin School of Medicine, head of the Department of Biomedical Engineering at the NUS College of Design and Engineering, previously developed an AI-based computational platform called IDentif.AI. The tool correlates input drug combinations with the corresponding cellular response via a mathematical relationship. Rather than using pre-existing information or data from other studies, the algorithm relies on a small batch of data generated for the current study.
“When harnessing digital approaches for healthcare, how we acquire data is at least as important as how much data we collect,” said Ho. “Treatment has different data requirements than diagnostics. Using carefully tested data points from actual experiments enables IDentif.AI to make actionable drug combination designs within days.”
That meant that the team only needed to test a strategic subset of 59 predetermined combinations and input those results into the platform to predict how all 6,000+ combinations would behave in vitro, said Aguado. The team followed up with validations.
“What we measured in the laboratory matched beautifully with what the algorithm predicted,” said Aguado. “That provided us confidence that the algorithm was actually predicting what we observe in the lab.”
Sex-specific synergy
The results showed that the drug combinations that were most effective for reducing stiffening activation in AVS were indeed different for male cells and female cells. While that might not have been a surprising result, it was surprising to the researchers how reproducible the results were.
What’s more, the top drug combinations were synergistic, meaning they had a combined effect greater than the individual effect of each drug. But that was only true when the male cells were treated with the best male combo or when the female cells were treated with the best female combo—not for sex mismatch cases. In other words, the synergies were sex-biased.
To the researchers, the strength of this proof of concept indicates that the sex differences could be leveraged to improve therapeutic outcomes in both male and female patients.
“Our work is part of a larger trend of moving toward precision medicine, where we’re trying to move away from the one-size-fits-all approach,” said Vogt.
For this study, the researchers used pig cells. According to Aguado, pig cells are a great model for human disease; many, but not all, of the signaling pathways are the same. Still, he added, it’s important to look at human cells, and his lab has already started doing so. In addition, the researchers are digging deeper into why these sex differences exist and whether the responses of the signaling pathways are driven by hormones or chromosomes or both.
One more takeaway from the study that Vogt hopes other researchers understand is to always be as transparent as possible in their own research and report the sex and source of biological materials they are using in their studies, even if not specifically looking at sex differences. That would help when looking back in meta-analyses and benefit future research.
The team also stressed that they only looked at biological sex: female pigs with XX chromosomes and male pigs with XY chromosomes.
“Gender identity in humans will also inform drug combinations. Our studies are suggesting that biological sex is also a potent contributor to defining what drug combinations a patient should receive,” said Aguado.
Paper: “Determining sex differences in drug combinations targeting aortic valve myofibroblast activation using an artificial intelligence derived platform.” Co-authors of the study include Brandon J. Vogt*, Megan Chavez, Peng Guo and Brian A. Aguado, UC San Diego; Peter Wang*, Edward Kai-Chow and Dean Ho, National University of Singapore.
*These authors contributed equally to this work.
This work was supported by the National Science Foundation Graduate Research Fellowship Program (NSF-GRFP DGE-2038238), Digital Medicine Translational Research Programme (A-0001319-00-00), The N.1 Institute for Health, the College of Design and Engineering at the National University of Singapore, the National Institutes of Health (R00 HL148542), the NIH Director’s New Innovator Award (DP2 HL173948), the Chan Zuckerberg Initiative Science Diversity Leadership Award and the American Heart Association (942253).
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.