Rare Science
UC San Diego scientists explore the hidden world of rare disease research, offering hope to patients and caregivers.
Published Date
Story by:
Share This:
Article Content
This story was published in the Spring 2023 issue of UC San Diego Magazine.
Medical conditions we are likely to never see, suffer from or even hear about receive little attention. Rare diseases, or those affecting fewer than 200,000 people in the U.S., are largely unknown because they are rare. But what rare diseases share with other, more common ailments is the depth and devastation of their severity.
The cause is often unknown. The prognosis is not good. Many rare diseases result from genetic mutations that fundamentally alter the nature of life and living. They consume the lives of both patients and caregivers. These diseases are characterized by profound disabilities and even premature death.
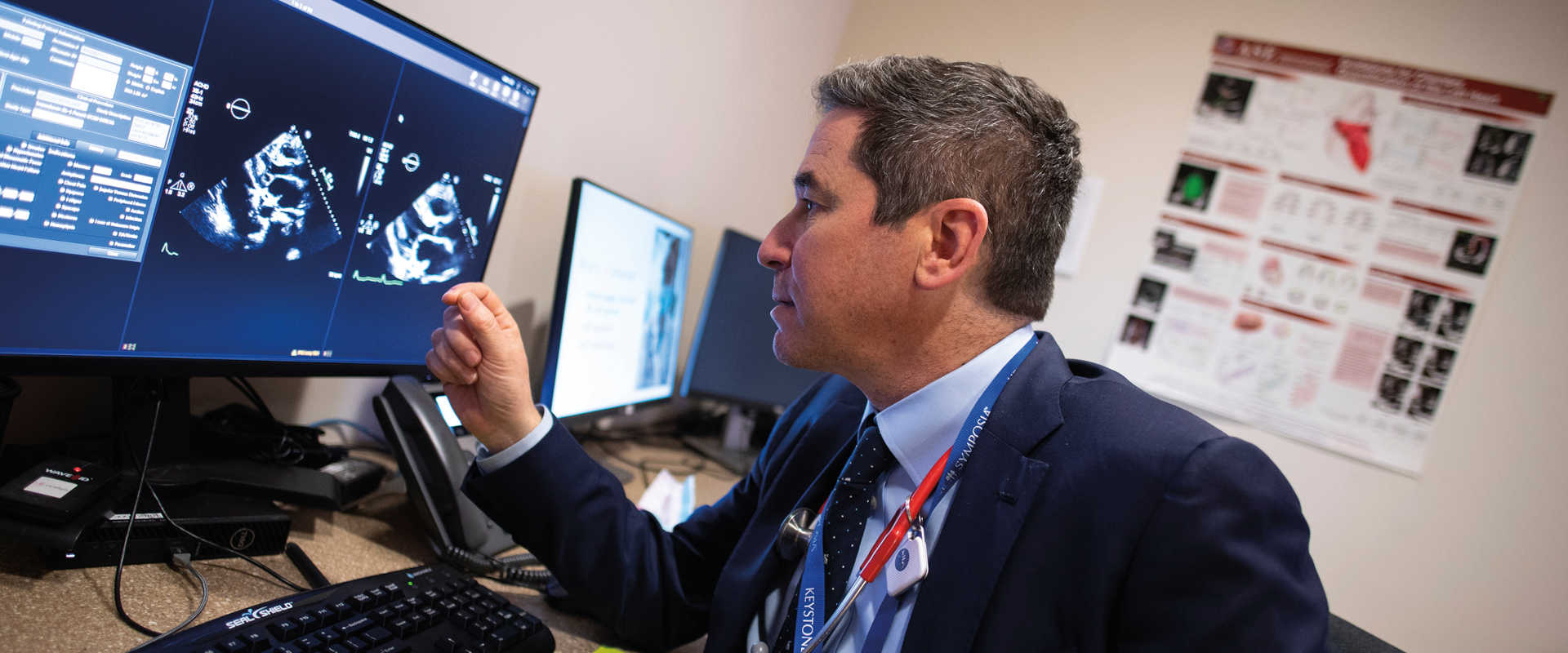
“Danon disease, an inherited condition involving progressive weakening of body muscles, particularly the heart, is a death sentence without treatment,” says Eric Adler, MD, a cardiologist and director of cardiac transplant and mechanical circulatory support at UC San Diego Health. “The current standard of care is a heart transplant or [death] by age 25.”
Danon disease is very rare — and the diagnosis is terrifying. There are an estimated 10,000 persons in the U.S. with the condition; most are undiagnosed. Other rare diseases foretell similar fates: lives defined by progressive physical and neurological degeneration that may eventually render patients unable to stand, walk, speak, eat or even breathe without drugs, devices and external aid. And their rarity makes these conditions notoriously difficult to study.

But that is changing.
UC San Diego has become a robust hub for rare disease research. And at UC San Diego Health, physicians treat a growing number of rare diseases, frequently through novel clinical trials, that are often untreatable elsewhere.
“To make this kind of work happen, it takes a commitment to both clinical excellence and research that is often only available at a major research university and affiliated academic medical center,” says Marcus Urey, MD, assistant professor of medicine at UC San Diego School of Medicine and cardiologist at UC San Diego Health.
For instance, in 2022, UC San Diego Health became the first health system in the nation to offer an injectable medication to treat patients with hereditary transthyretin amyloidosis, a rare condition that causes a buildup of protein deposits and leads to heart dysfunction and failure. Before the breakthrough injections debuted, treatment required lengthy, frequent intravenous infusions.
Heart of the Matter
It's a matter of numbers that makes rare diseases difficult to study and treat.
“People with a specific rare disease are relatively scarce, at least compared to people with conditions like cancer, diabetes and more common forms of heart disease,” says Adler. “It’s the classic conundrum. Effective treatments are limited or don’t exist because nobody has done the research and clinical work.” Additionally, doctors might not recognize the disease, so patients may receive a diagnosis after it is too late for treatment.
A decade ago, Adler became interested in Danon disease after treating a man in his 20s who needed a heart transplant although doctors didn’t know why the patient was so ill. “Tissue analysis revealed that he had Danon disease, which was different from his original diagnosis,” says Adler. “Unfortunately, he was too sick for a transplant and died. I didn’t know much about the condition then, but I wanted to learn more, especially the underlying mechanisms that caused it.”
Within months, Adler treated a second patient with Danon disease who also died. Two cases in less than four months galvanized Adler’s urgency to understand the disease and find a remedy. In the ensuing years of research, Adler and colleagues developed a gene therapy that adds a modified gene to patients to repair muscle dysfunction caused by mutations in a gene called LAMP2, a cause of Danon disease.
In 2019, Adler launched a five-year national trial co-led by Barry Greenberg, MD, a transplantation cardiologist and professor of medicine at UC San Diego School of Medicine.
Today, Adler spends up to half of his time focused on Danon disease, overseeing both a specialized lab and a clinical care team. The program at UC San Diego Health, which also includes a clinical trial chronicling the natural history of Danon disease, attracts patients from around the world.
“It’s the classic conundrum. Effective treatments are limited or don’t exist because nobody has done the research and clinical work.”

Finding Hope
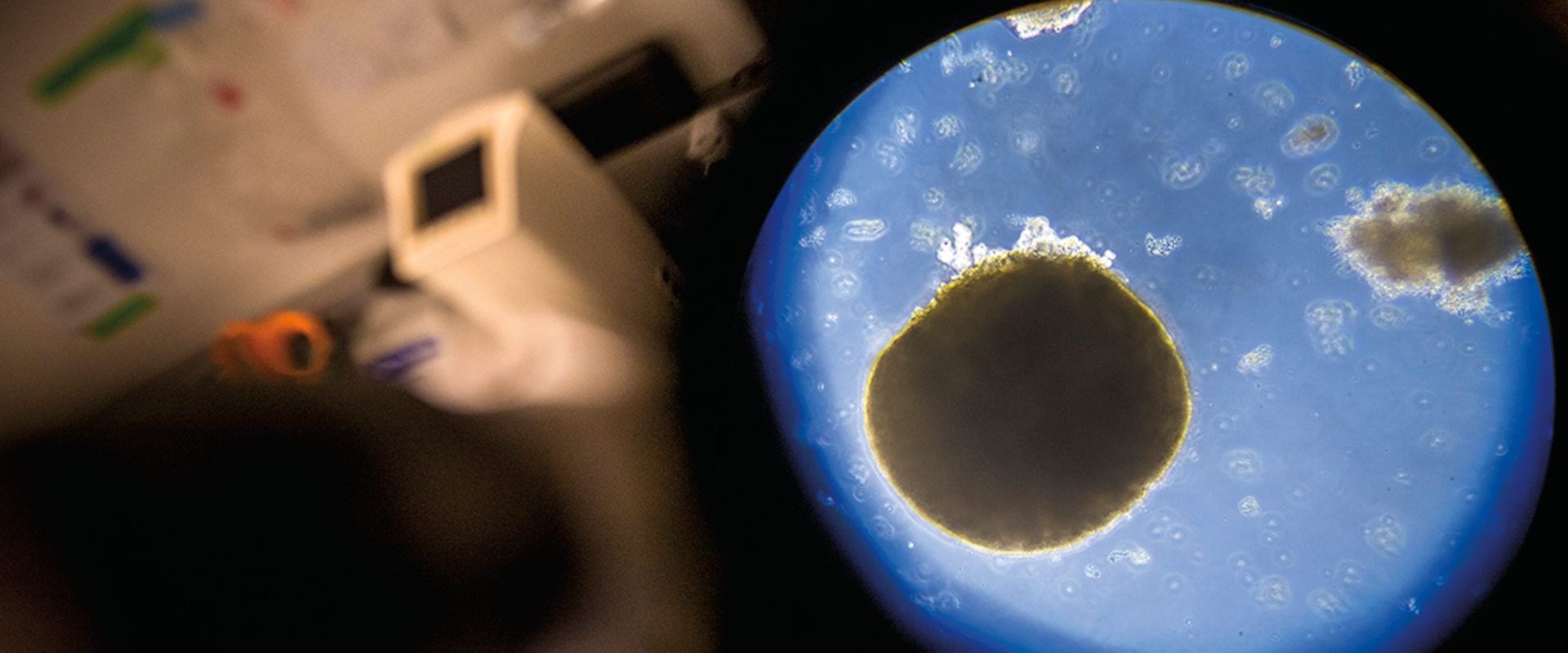
Stephanie Cherqui, PhD, professor of pediatrics at UC San Diego School of Medicine, has long been fascinated by human genetics. After enrolling in the prestigious Université Paris Diderot, she turned her attention to human genetic diseases and, specifically, a condition called cystinosis.
Cystinosis is caused by a genetic mutation of the CTNS gene, resulting in an abnormal accumulation of the amino acid cystine in organs and tissues, including the kidneys, eyes, pancreas and brain. Without treatment, patients usually develop end-stage kidney failure and die prematurely. Standard care involves drugs used to lower cellular cystine levels, but it only delays disease progression. Life expectancy is approximately 10 years, but with current treatments, patients may survive to middle age.
During Cherqui’s defense of her doctoral thesis, a professor complimented her work but wondered why Cherqui chose to labor on such a rare disease affecting one in 100,000 to 200,000 people.
“For the parents who have a child who is dying from a rare disease, there is no hope,” says Cherqui. “Fortunately, some researchers work on these diseases to find a treatment.”
Cherqui and colleagues have since identified the gene mutation that causes cystinosis and created a mouse model of the disease. Like Adler’s work, these efforts have led to a potential treatment. In 2020, Cherqui launched a Phase I/II clinical trial using gene and stem cell therapy. She has recruited six patients. The trial will run for five years, and early results have been encouraging.
“For the parents who have a child who is dying from a rare disease, there is no hope. Fortunately, some researchers work on these diseases to find a treatment.”

Brain Power
Alysson R. Muotri, PhD, professor of pediatrics and cellular and molecular medicine and a member of the Sanford Stem Cell Institute at UC San Diego Health, possesses an intimate understanding of patients with rare diseases.
His own child carries a genetic mutation that causes a neurological disorder, a personal motivation that helped drive Muotri’s passion to look deeper into the cellular and molecular manifestations of rare diseases.
“Initially, I started working with genes that cause rare neurological conditions because I was studying the impact of perturbation of these genes during human neurodevelopment,” says Muotri. “As the work progressed, I started to connect with families and support groups looking for treatments, and so over time, my interest shifted from pure basic biology to more translational efforts.”
Those efforts typically involve human brain organoids — three-dimensional, artificially grown masses of cells that can mimic functional aspects of the brain. For example, Muotri has created nine-month-old brain organoids that generate electrical signals similar to patterns produced in the developing brains of premature babies. Organoids allow scientists such as Muotri to test hypotheses and experimental treatments earlier, without human participants, to create stronger and more nuanced foundations for subsequent clinical trials. Muotri and colleagues have created brain organoids mimicking the dysfunction associated with a number of rare neurological disorders, including an array of syndromes falling within autism spectrum disorder (ASD).
Through his research with organoids, Muotri has shown that molecular, cellular and network defects in Pitt-Hopkins syndrome, a condition characterized by intellectual disabilities, developmental delays, seizures and certain features of ASD, are reversed by restoring expression of the TCF4 gene. “The next step before a clinical trial is to make sure the viral vector does not cause any kind of eventual toxicity. We are investigating this in animal models,” says Muotri. “We hope the strategy will work in humans and lead to a significant improvement of the condition with no major side effect.”
“As the work progressed, I started to connect with families and support groups looking for treatments, and so over time, my interest shifted from pure basic biology to more translational efforts.”
Uncommon Challenges
While there are numerous challenges in studying rare diseases, Adler believes it’s easier now than ever before.
“Scientific and technological advances have made the research quicker and more effective,” he says. “We have more tools to probe and understand the genetics of disease. Clinical trials can be smaller. Drugs can be approved faster, too.”
And yet obstacles still remain.
“It is still a constant challenge to convince agencies, stakeholders and the public that rare disease research is critical and requires sufficient funding,” says Cherqui.
For instance, the discoveries made while studying rare diseases can be applied to other, more prevalent and visible disorders and diseases.
Rare disease research can be lonely work, but there is a sharper sense of purpose and community that comes from working with others on a cure for a disease that affects so few people.
Cherqui says these diseases create tight-knit communities of patients and families who are tireless in their advocacy for more research and better treatments. “In many ways, they are our best supporters,” she adds. “They keep us going.”
Muotri says there is a great strength among these communities, and he tries to help by connecting them together. For example, in 2012, he launched the Tooth Fairy Project to recruit families with children with ASD to mail him one of their baby teeth. Muotri’s team has extracted genetic information from more than 3,000 donated teeth to identify potential new autism candidate genes. And more recently, he created the Tismoo.me app, an online social network to inform and connect families around the world who have members afflicted with a range of rare neurological disorders.
And perhaps someday, it will render a rare disease even rarer.
To support UC San Diego Health, visit giving.ucsd.edu/health-medicine
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.





