Quenching Water Scarcity with a Good Pore
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
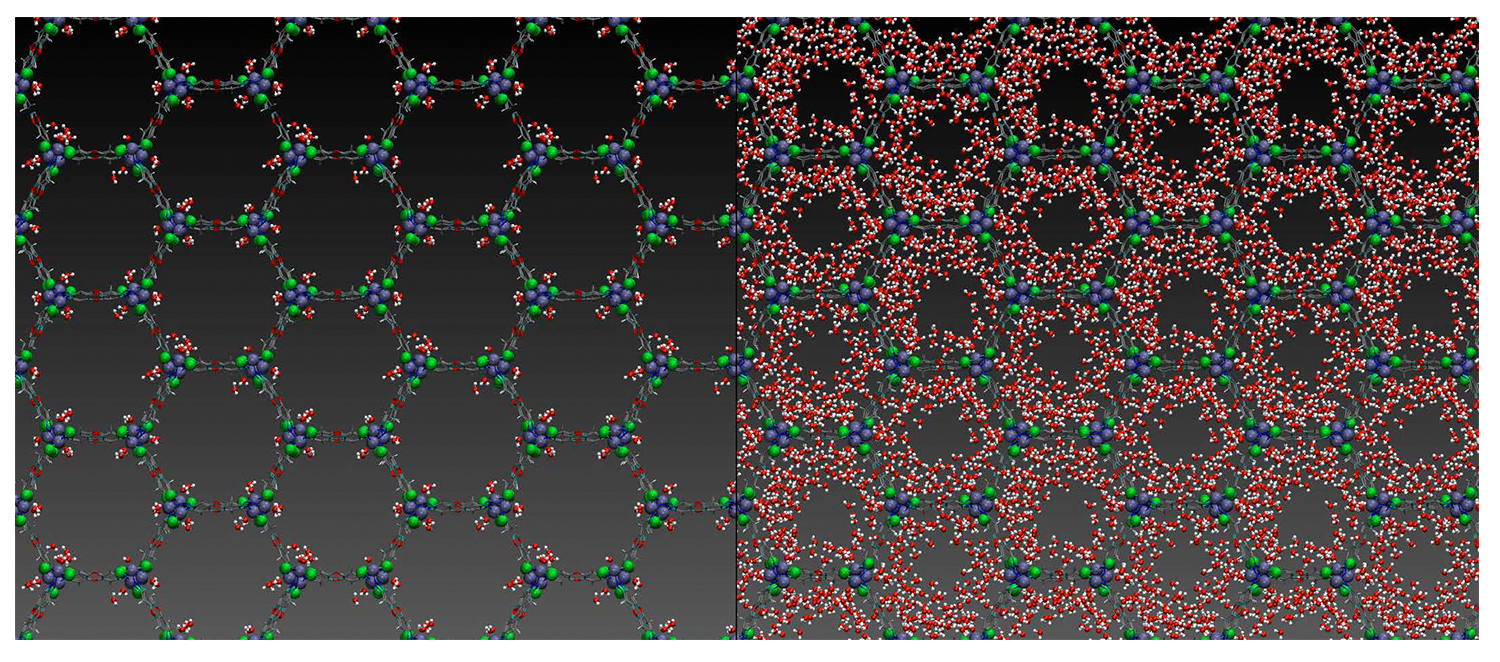
MOF pores at low (left) and high (right) relative humidity. Figure by Kelly Hunter, Paesani Research Group
Picture this: Desert. High heat, low humidity. No water. What’s a thirsty person living amidst climate change to do? Potentially, pull out a mechanism studied by UC San Diego’s Paesani Research Group and turn air into water with a good “pore.”
On the landscape of chemistry, a pore is part of a metal-organic framework referred to as a MOF. According to research results recently published in Nature Communications, UC San Diego Professor of Chemistry and Biochemistry Francesco Paesani and PhD Student Kelly Hunter, in collaboration with the Dincă Group at MIT, linked theory and experiment to shed light on how water interacts with MOFs and move closer to developing materials that address the challenge of global water scarcity.
“All of our comparisons between theory and experiment showed really good agreement for a system like this, so to our knowledge this is some of the best agreement we’ve seen,” said Hunter, who ran the simulations and computations for the project.
The third-year graduate student explained that the research findings elucidated the mechanism of pore filling — the process of the adsorption of water inside a MOF — from a few water molecules to the many that fill it. Granted, a MOF crystal is small — much smaller than a grain of sand or a flake of powder — but the team was able to ascertain how water adsorbs inside the tiny space, gaining insight into its structural and dynamical properties.
Simulation of water inside the MOF pore at a low relative humidity. Video by Kelly Hunter, Paesani Research Group
“Now, we know where the molecules bind,” said Paesani, who explained that the properties of materials, like MOFs, can exploit temperature differences throughout the day. “MOFs can adsorb water as a vapor at night when the temperature is lower and release it during the day as condensed droplets—a process that converts water vapor from air into drinkable water.”
According to Paesani, since the researchers now know where the molecules adsorb, the next step would be to make materials with new structures on a computer and perform simulations that would closely approximate the behavior of water.
“Researchers can do it in their labs faster and cheaper, so we can make it from many different structures. Then we can screen those structures for the ability to capture water from air and identify the MOFs that are better at doing that,” explained Paesani. “Then we can go back and determine a structure that allows the harvesting of water from air at low relative humidity. This is going to be a big achievement because if you are in the desert, you are going to get water from the air.”
Matching simulations with experiments is key to the design of new MOFs with better adsorption properties.
“We use computer simulations as a nanoscope to look inside a MOF, which is an extended three-dimensional structure with many small pores,” described Hunter. “This is what makes metal-organic frameworks really unique in that you have these small pores that are periodic in space and provide a very large surface area so MOFs can adsorb various liquids and vapors. Therefore, they can be used for a wide variety of applications and processes.”
Hunter said that being able to put together and perform the simulations and end up with a fine-tuned picture of what’s going on at the molecular level inside a pore, on such a small scale, was rewarding and exciting.
“We now know that our simulations are able to accurately reproduce experiments; therefore, we can use them to contribute to the development of new technologies that aim to address water scarcity, which is an urgent problem in today’s world,” she said.
This research was supported by a CAREER grant from the National Science Foundation (DMR-1452612), the Martin Family Fellowship for Sustainability, the Abdul Latif Jameel World Water and Food Security Lab, the U.S. Department of Energy (grant no. DE-SC0019333), the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (grant no. ACI-1053575 under allocation TG-CHE110009) and the Air Force Office of Scientific Research (grant no. FA9550-16-1-0327).
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



