Putting a Brake on Tumor Spread
By:
- Scott LaFee
Published Date
By:
- Scott LaFee
Share This:
Article Content
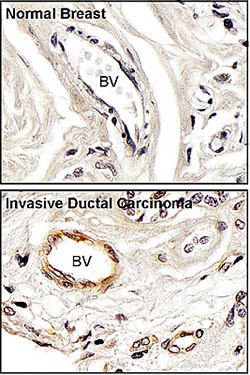
Normal breast tissue and invasive ductal carcinoma stained brown with antibodies to activated FAK. Blood vessels are indicated by BV.
A team of scientists, led by principal investigator David D. Schlaepfer, PhD, a professor in the Department of Reproductive Medicine at the University of California, San Diego School of Medicine, has found that a protein involved in promoting tumor growth and survival is also activated in surrounding blood vessels, enabling cancer cells to spread into the bloodstream.
The findings are published in this week’s online issue of The Journal of Cell Biology.
Blood vessels are tightly lined with endothelial cells, which form a permeability barrier to circulating cells and molecules. “Our studies show that pharmacological or genetic inhibition of the endothelial protein focal adhesion kinase, or FAK, prevents tumor spread by enhancing the vessel barrier function.”
The researchers found that selective FAK inhibition within endothelial cells prevented spontaneous tumor metastasis without alterations in tumor size. Schlaepfer, with colleagues at the UC San Diego Moores Cancer Center, is exploring whether inhibiting targets like FAK, which has important regulatory functions in both tumor cells and blood vessels, might provide a dual mechanism for preventing both cancer growth and spread.
Using mouse models of breast, ovarian and melanoma tumors, first author Christine Jean, PhD, showed that FAK activity was elevated in the blood vessels surrounding tumors, compared to normal tissue. FAK modifies the function of other cellular proteins, and researchers identified a previously unknown FAK target: a protein called vascular endothelial cadherin (VE-cadherin) that helps endothelial cells fasten tightly together. When modified by FAK, VE-cadherin complexes fall apart and blood vessels become leaky. Inactivating FAK within endothelial cells prevented this unwanted permeability and helped block the ability of tumor cells to pass through endothelial cell barriers.
Schlaepfer said the research has major clinical implications: Metastasis – or the spread of a cancer from its originating site to other parts of the body – is responsible for 90 percent of cancer-related deaths. “This fact alone underscores the need for a better mechanistic understanding of the metastatic process,” Schlaepfer said. He noted that several FAK-inhibitors are currently being tested in clinical trials.
Co-authors include Xiao Lei Chen, Isabelle Tancioni, Sean Uryu, Christine Lawson, Kristy K. Ward and Nichol L.G. Miller, UC San Diego Moores Cancer Center; Ju-Ock Nam, Kyungpook National University, Korea; Colin T. Walsh, Binomics, San Diego; Majid Ghassemian, UCSD Department of Chemistry and Biochemistry; Patrick Turowski, University College London; Elisabetta Dejana, University of Milan; Sara Weis and David A. Cheresh, Department of Pathology, UC San Diego Moores Cancer Center.
Funding for this research came, in part, from the National Institutes of Health (grants RO1 HL093156, RO1 CA102310, and R37 CA50286), Italian Association for Cancer Research and The European Research Council, American Heart Association, Susan G. Komen for the Cure, Canadian Institutes of Health Research, Ruth Kirschstein National Research Service Award and Nine Girls Ask?
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



