New Insights on Pelvic Floor Damage after Vaginal Birth, and New Directions for Treatment
UC San Diego bioengineers and physician-scientists are working together to improve treatment for pelvic floor disorders that impact close to a quarter of women in the U.S.
Story by:
Published Date
Article Content
University of California San Diego researchers are leading a team reporting new insights in Science Translational Medicine on pelvic floor muscle (PFM) dysfunction, which is one of the key risk factors for pelvic floor disorders which impact close to a quarter of women in the U.S. Pelvic floor disorders are a set of conditions that include pelvic organ prolapse and urinary and fecal incontinence that have a strong association with vaginal childbirth.
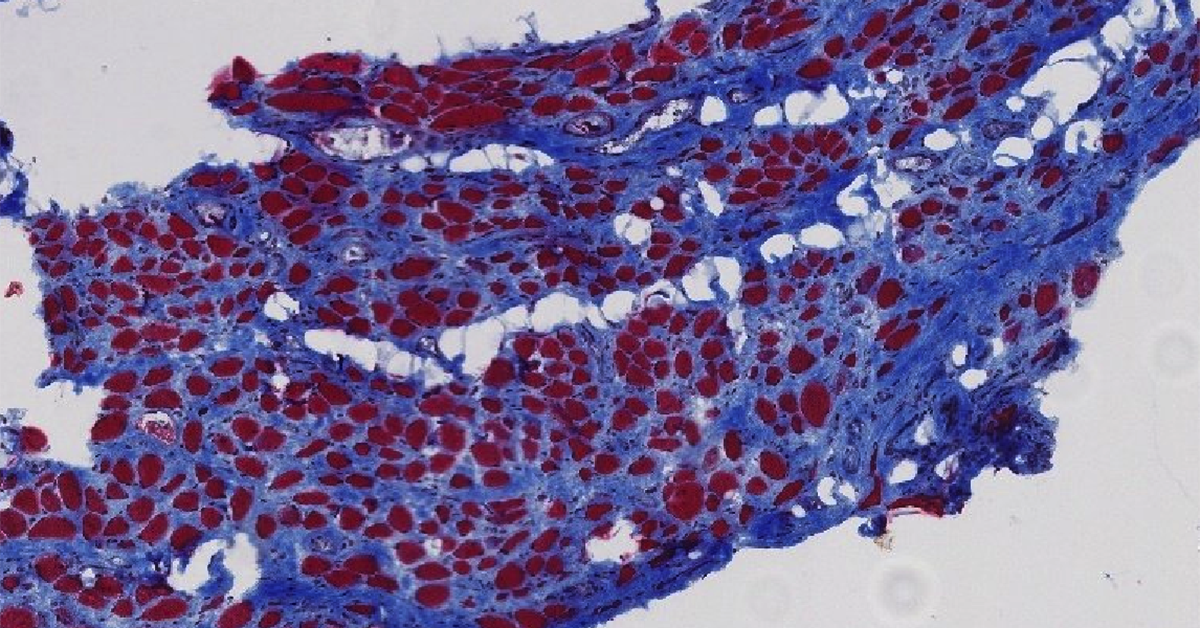
The UC San Diego team reports new direct evidence of both atrophy and fibrosis in the skeletal muscles of the pelvic floor of women with symptoms of pelvic organ prolapse. In addition, the team showed that in a well-established rat model for vaginal birth injury in humans, the pelvic floor muscles of the female rats sustained the same kinds of negative muscle transformations – atrophy and fibrosis – seen in the pelvic floor muscle biopsies of women. Finally, the UC San Diego team treated rats subjected to simulated birth injuries with an acellular injectable skeletal muscle extracellular matrix (ECM) hydrogel either at the time of or four weeks after simulated birth injury. They found that the administration of the hydrogel reduced the negative impact of the simulated birth injury on the rat pelvic floor muscles.
The work, published in the August 02, 2023 issue of Science Translational Medicine, is part of a larger effort from this team of bioengineers, physician-scientists and basic scientists who are working together to advance understanding, treatment and prevention of pelvic floor muscle dysfunction in humans.

Pelvic floor muscle damage
“Understanding the natural pelvic floor muscle response after birth injury is crucial for developing and applying regenerative medicine approaches,” said Pamela Duran, PhD, the first author on the paper. Duran recently completed her PhD in bioengineering at the University of California San Diego Jacobs School of Engineering. Duran is currently a postdoctoral researcher at the University of Michigan. “In this new work, we first investigated the pelvic skeletal muscles' short- and long-term responses after birth injury using a rat preclinical model. With these findings, we rationalized applying a cell-free biomaterial to prevent and treat the pathological changes of the pelvic floor muscle after simulated birth injury. The use of a low-cost and minimally invasive biomaterial is crucial for the clinical translation of this regenerative medicine approach to counteract the negative alterations of the pelvic floor muscles.”
In the new paper, the team presents new tissue-level research demonstrating that for cis-women who have given birth vaginally and have symptoms of pelvic organ prolapse, their pelvic floor muscles show the damage signs of atrophy and fibrosis – which includes the excess buildup of collagen. This new direct evidence of both atrophy and fibrosis in the skeletal muscles of the pelvic floor of women with symptoms of pelvic organ prolapse is an important step toward developing strategies to either prevent damage or aid recovery after damage occurs. This tissue-level research included samples from age-matched human cadavers as well as women undergoing surgery for pelvic organ prolapse.

Insights from a preclinical model of birth injury
“Current clinical and preclinical strategies for treating damaged pelvic floor muscles have focused on late stage treatments that are suboptimal for patients and do not address the underlying causes of muscle damage. In this preclinical research, we have shown that injecting a hydrogel directly into muscle tissue of the pelvic floor offers a potential method for encouraging and accelerating the natural healing process. In particular, we see the possibility of muscle fiber restoration rather than the unhealthy buildup of collagen,” said Karen L. Christman, a professor in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering.
Professor Christman is a cofounder, consultant, board member, and holds equity interest in Ventrix Bio Inc. and Kairos Technologies Inc. These two companies are working to translate biomaterial technologies into the clinic for applications including efforts to encourage regeneration of heart tissue and improved heart functionality after a heart attack, and prevent adhesions after cardiothoracic surgery. Learn more: This Injectable Biomaterial Heals Tissues from the Inside Out. Christman is also the Associate Dean for Faculty Affairs and Welfare at the UC San Diego Jacobs School of Engineering, where she holds the Pierre Galletti Endowed Chair for Bioengineering Innovation.
In the Science Translational Medicine study, the team showed that in a well-established rat model for vaginal birth injury in humans, the pelvic floor muscles of the female rats sustained the same kinds of negative muscle transformations – atrophy and fibrosis – seen in the pelvic floor muscle biopsies of the women who have given birth and went on to develop pelvic floor prolapse. This finding serves to validate that additional research using this rat model of birth injury will be relevant for understanding and potentially finding better ways to treat or even prevent pelvic floor dysfunction and the associated pelvic floor disorders in women.

A new approach for preventing or healing pelvic floor muscles
“Pelvic skeletal muscles’ birth injury and subsequent degeneration is a key risk factor for pelvic floor disorders that negatively impact lives on millions of women worldwide. Unfortunately, we know very little about tissue-level changes that take place in these important muscles as a result of maternal birth injury. The findings of our study are important because without understanding what goes wrong in pelvic floor muscles, we can’t develop effective strategies to treat these important components of the pelvic floor,” said Marianna Alperin, M.D., M.S. Professor of Obstetrics, Gynecology, and Reproductive Sciences, Professor of Urology, and Fellowship Research Director in the Division of Urogynecology & Pelvic Reconstructive Surgery at University of California San Diego. Dr. Alperin is also affiliated with the Sanford Consortium for Regenerative Medicine.
In the study, the team showed that a tissue-specific cell-free pro-regenerative biomaterial, similar to the material invented at UC San Diego that is currently in clinical trials for helping to improve healing of heart tissue after heart attack, could serve as a new approach for helping to prevent or heal pelvic floor muscles injured during childbirth. In particular, the UC San Diego team treated rats subjected to simulated birth injuries with an acellular injectable skeletal muscle extracellular matrix (ECM) hydrogel either at the time of or four weeks after simulated birth injury. They found that the administration of the hydrogel reduced the negative impact of the simulated birth injury on the rat pelvic floor muscles. This new work highlights the need for further investigations of this promising biomaterial for the prevention of pelvic floor muscle dysfunction after birth injury.
“Currently available preventative or therapeutic strategies are extremely limited and do not include regenerative approaches. While cell-based therapies are promising in many areas of medicine, they are associated with many hurdles, including substantial costs. In contrast, the biomaterial tested in our study does not contain any cells and is therefore very safe and is low-cost. Investigating what goes wrong in the pelvic skeletal muscles and developing pragmatic approaches to overcome these negative changes is very important for improving women’s health,” said Alperin.
Paper
“Proregenerative extracellular matrix hydrogel mitigates pathological alterations of pelvic skeletal muscles after birth injury” published by Science Translational Medicine on August 02, 2023.
Authors
Pamela Duran, Francesca Boscolo Sesillo, Mark Cook, Lindsey Burnett, Shawn A. Menefee, Emmy Do, Saya French, Gisselle Zazueta-Damian, Monika Dzieciatkowska, Anthony J. Saviola, Manali M. Shah, Clyde Sanvictores, Kent G. Osborn, Kirk C. Hansen, Matthew Shtrahman, Karen L. Christman, and Marianna Alperin
Institutional Affiliations
- Shu Chien-Gene Lay Department of Bioengineering, University of California San Diego
- Sanford Consortium for Regenerative Medicine, University of California San Diego
- Department of Obstetrics, Gynecology, and Reproductive Sciences, Division of Female Pelvic Medicine and Reconstructive Surgery, University of California San Diego
- Department of Integrative, Biology and Physiology, University of Minnesota, Minneapolis
- Department of Obstetrics and Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, Kaiser Permanente
- Department of Biology, University of California San Diego
- Department of Biochemistry and Molecular Genetics, School of Medicine, University of Colorado
- Department of Physics, University of California San Diego
- Center for Veterinary Sciences and Comparative Medicine, Division of Comparative Pathology and Medicine, University of California San Diego
- Department of Neurosciences, University of California San Diego
Funding
Funding for this work was provided by the NIH/NICHD [R21HD094566 (M.A. and K.L.C.), R01HD092515 (M.A.), R01HD102184 (M.A. and K.L.C.)], Galvanizing Engineering in Medicine award supported by the NIH grant UL1TR001442 of CTSA and by funds provided by the University of California San Diego Chancellor. P.D. was supported through the NIAMS T32 Predoctoral Training Grant (T32AR060712) and an NIH/NICHD F31 Predoctoral fellowship (F31HD098007). L.B. received support from June Allyson Memorial Fund Research Award, American Urogynecologic Society and Ellis Wyer Foundation Grant, University of California San Diego, Division of Female Pelvic Medicine and Reconstructive Surgery.
Competing interests information
Karen L. Christman, Marianna Alperin, and Pamela Duran are inventors on a patent (US11376346B2, Extracellular Matrix for Treating Pelvic Floor Disorders and Skeletal Muscle Degeneration) related to this work.
Karen L. Christman is a cofounder, consultant, board member, and holds equity interest in Ventrix Bio Inc. and Kairos Technologies Inc,. and is a consultant for Coloplast. Christman reports an editorial stipend from npj Regenerative Medicine.
Marianna Alperin reports an editorial stipend from American Journal of Obstetrics and Gynecology.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.