Comet Supercomputer Used to Illustrate Methane Storage Applications
New findings could lead to carbon-neutral chemical fuels for vehicles
By:
- Kimberly Mann Bruch
- Cynthia Dillon
Media Contact:
- Kimberly Mann Bruch - kbruch@ucsd.edu
- Cynthia Dillon - cdillon@ucsd.edu
Published Date
By:
- Kimberly Mann Bruch
- Cynthia Dillon
Share This:
Article Content
Porous carbon is a well-established class of materials with significant potential in wide-ranging applications – from water purification and gas separation to energy storage devices and thermal insulation. A class of first-year graduate students is also full of potential – especially if those among them have already been part of an international research team whose work could impact technology needed for reduced-carbon or carbon-free chemical fuels for vehicles.
Rylan Rowsey, a first-year graduate student in chemistry at UC San Diego, attended Montana State University, Bozeman (MSU) as an undergraduate and worked with an international team of researchers who used Comet at the San Diego Supercomputer Center (SDSC), located at UC San Diego, to complete a detailed study of a particular sub-group of porous carbon called zeolite-templated carbon (ZTC) as a gas storage material.
“The computational chemistry skills and tools I learned undergoing this work are invaluable to my growth as a researcher, and now that I am beginning graduate school, I feel that I am coming in prepared with a unique skill set,” said Rowsey.
The work to which Rowsey referred is the research team’s use of computational modeling of ZTC structural units (referred to as maquettes – small models of surface sites of interest) to evaluate methane gas binding energy as a function of chemical composition. Led by Nicholas Stadie, an assistant professor of physical chemistry and materials science at MSU and Robert Szilagyi, an MSU associate chemistry and biochemistry professor, the team’s study results were published recently in the Journal of Physical Chemistry A.
“From a comprehensive set of calculations, we clearly identified the preference of methane toward nitrogen-substituted adsorption sites,” said Szilagyi. “The theoretically estimated binding energies and the experimentally measured heat of adsorption values are in good agreement, validating the use of computational chemistry as a tool to design new porous carbon materials for methane storage applications – a key bridging technology to reduced-carbon or carbon-free chemical fuels for vehicles.”
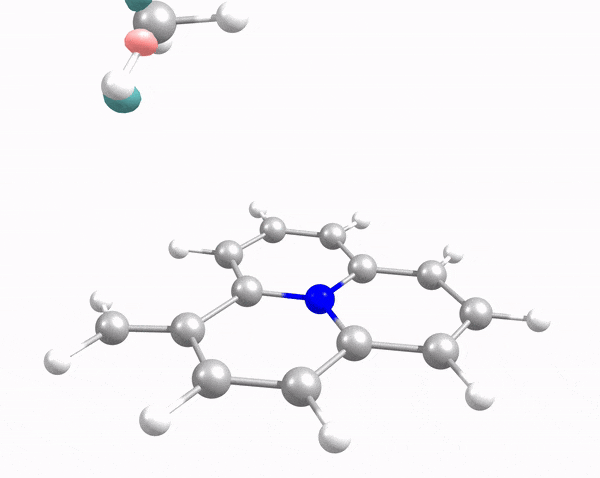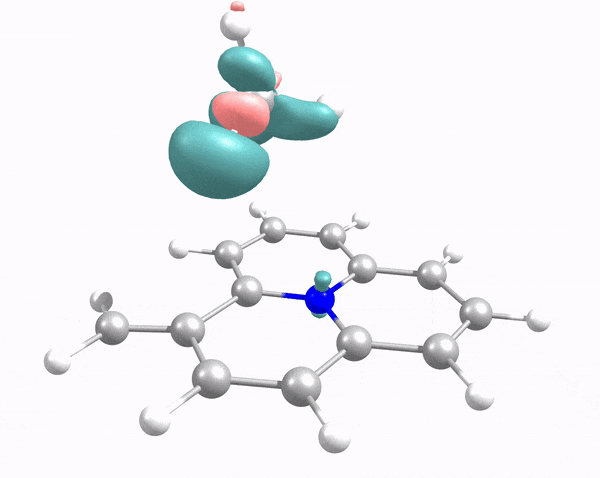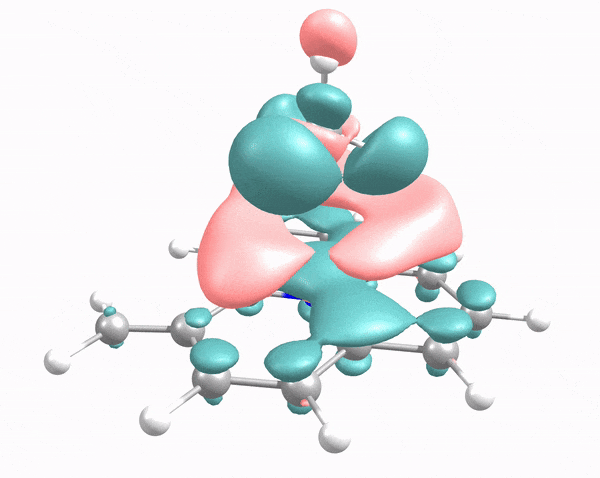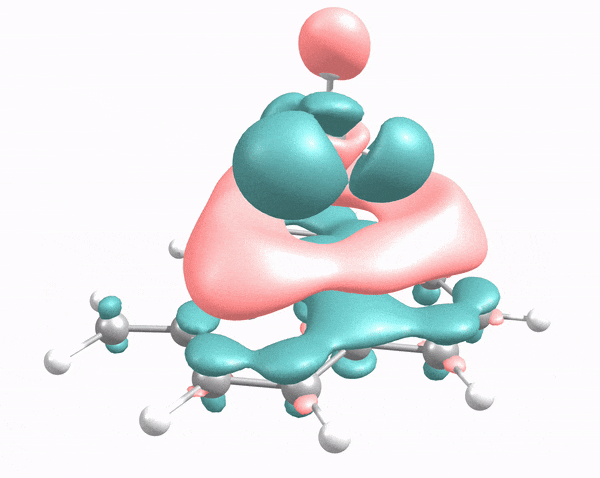
This animation illustrates how an arriving methane molecule (adsorbate) and carbon surface model (adsorbent) mutually modify each other’s electron density. Here, the methane molecule “lander” touches down at the carbon “moon surface,” “kicking up dust” (i.e., electron density, shown in pink and green) in the process. Credit: Robert K. Szilagyi, Montana State University
Why It’s Important
Computer-aided rationalized design of new materials provides an efficient way to develop new or optimize existing energy storage and conversion technologies. Instead of a traditional trial-and-error approach, computer modeling of chemical processes reduces product-to-market times, minimizes chemical waste and maximizes the use of human resources and brain power.
Specifically, methane storage and conversion to energy or to chemical feedstocks without greenhouse gas emissions is a long overdue target for petrochemical industries. Computer modeling-based materials design allows for simulation of every step of methane storage or conversion at the atomic-scale, which provides a clear understanding of potential inhibiting steps and provides insights into how to mitigate harmful emissions and toxic chemical waste.
Showcasing Nitrogen-Doping of Carbon Materials
While the experimental results utilized in the computational work were obtained in Stadie’s laboratory, Stadie and Szilagyi next demonstrated that experimentalists can use simulation results to guide their strategies in synthesizing nitrogen-doped carbon materials for methane storage and delivery. The computational results clearly documented the benefit of focusing on nitrogen-doping of carbon materials when it comes to methane storage. Additionally, Szilagyi said that this work serves as a map of the chemical accuracy of simplified, but realistic models of porous carbon surfaces for many other problems.
“We were really surprised by the clear and consistent preference for methane toward nitrogen-substituted porous carbon models, over both the unsubstituted and the boron-substituted models,” said Szilagyi.
“This may not seem like a large effect to many, but this was just the size of effect we were expecting,” added Stadie. “This effect has very important implications for methane storage at ambient temperature.”
How Supercomputing Helped
While the team had access to a modest laboratory workstation cluster and MSU’s Hyalite high- performance computing system, accessing Comet allowed for the speedy completion of especially intense computational experiments that would otherwise have been very challenging. The team was able to run potential energy surface mapping calculations where each grid point was calculated at the appropriate level of theory without truncations and simplifications.
“The additional computational resources allowed us to complete a more thorough study, including computational control and blank simulations, which are mandatory for experimental work, but are often omitted in theoretical studies,” said Szilagyi.
“The experimentalist’s approach to computational chemistry has great potential to elevate theoretical models to work directly alongside observations from our laboratory,” continued Stadie. “The successful combination of theory and experiment elevates the impact of computations in rationalizing experiments and generating experimentally testable hypotheses.”
What’s Next?
The approach developed in the first two years of this work has direct implications for the ongoing experimental research directions in the Stadie laboratory, from assisting with screening synthetic conditions, to down-selecting gas adsorption studies and predicting new technological applications beyond gas storage. The research team has already started evaluating other molecular substitutions, exploring larger molecular models with oxygen-containing functional groups, considering other adsorbates, and scaling computational methods up to larger, periodic systems, just to name a few.
In addition to Rowsey and MSU graduate student Erin Taylor, Stadie and Szilagyi collaborated with computational soft-materials scientist Stephan Irle from Oak Ridge National Laboratory. Additional collaborators included Prof. Hirotomo Nishihara from Tohoku University in Japan and scholars Tamas Szabo (Hungary), Eva Scholtzova (Slovakia), Amrita Jain (Poland), and Monica Michalska (Czech Republic).
“Considering I am going to UC San Diego, I may even be able to work-in some time on Expanse (the new supercomputer at SDSC) in my new research,” said Rowsey.
This research was supported by the U.S. Department of Energy’s Office of Energy Efficiency and Renewable Energy (EERE) under the Hydrogen and Fuel Cell Technologies and Vehicle Technologies Offices (grant no. DE-EE0008815). Support for the analysis of theoretical results was provided by the U.S. Department of Energy Fossil Energy and Carbon Management Program, Advanced Coal Processing Program, C4WARD project. Additional support was provided by the American Chemical Society Petroleum Research Fund and the MSU Undergraduate Scholars Program. Computation on Comet was supported via XSEDE, which is supported by National Science Foundation (grant no. ACI-1548562). Additional computations were carried out using the Hyalite HPC System, operated and supported by the University Information Technology Research Cyberinfrastructure at Montana State University.
Share This:
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



