Fresh Sea Spray Turns ‘Sour’ after Being Airborne
Small aerosol particles are more acidic than the ocean within minutes, carrying implications for climate change and air quality
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
Video of a crashing wave and sea spray in the hydraulics lab wave flume at Scripps Institution of Oceanography. Video created by Victor Or.
Aerosols are everywhere. These tiny liquid or solid particles populate the atmosphere, emerging from natural and artificial sources like volcanoes and oceans, and fossil fuels and agriculture. Ranging in size from less than the width of the smallest viruses to roughly the diameter of a human hair, aerosols are mighty in their impact on climate and human health.
Aerosols also can be described as warming, as in dark soot, or cooling, as in sea spray. UC San Diego Distinguished Professor of Chemistry and Biochemistry Vicki Grassian and graduate student Kyle Angle studied the cool version, showing that sea spray aerosols become more acidic within two minutes of their creation.
Sea spray aerosols are born when bubbles burst and waves crash at the ocean’s surface. Before this study scientists thought that the watery particles, once produced, took their time—days or weeks—to turn “sour.” But Grassian and Angle, along with a team of researchers from the Center for Aerosol Impacts on Chemistry of the Environment (CAICE), including researchers from UC San Diego, Colorado State University, University of Wisconsin-Madison and UC Davis, demonstrated that aerosol acidity also depends on size—smaller particles are more acidic than larger ones.
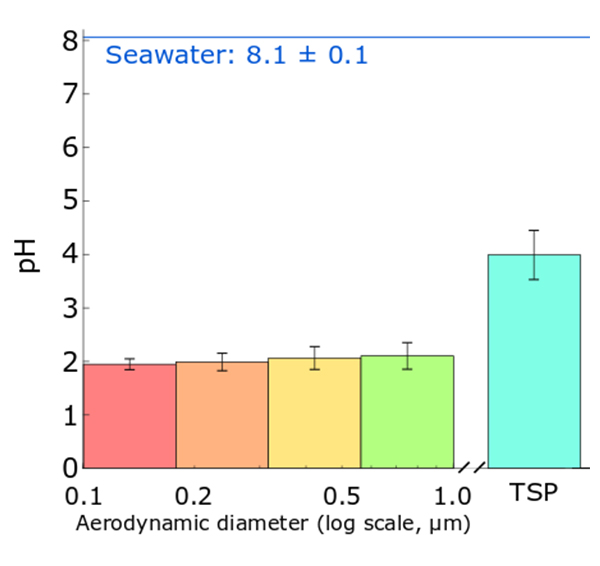
Graph showing how sea spray aerosols are more acidic than seawater. “pH” is a measure acidity, with a decrease of one pH unit meaning an increase in acidity by a factor of 10. The four bars on the left show the pH of small aerosols, and “TSP” refers to “Total Suspended Particles,” which is mainly large aerosols. Image adapted from Angle et al. Proceedings of the National Academy of Sciences, USA, 2020.
“The smallest particles become 100,000 times more acidic than the ocean within two minutes,” said Angle, first author of the paper published online in the journal Proceedings of the National Academy of Sciences. “I expected this to happen for particles that had been interacting with pollution for days, but seeing it for fresh sea spray was an interesting finding.”
The finding, according to Angle, is chemically important because the properties and reactivity of aerosols change with particle acidity. For example, the harmful pollutant sulfur dioxide is oxidized to sulfate more quickly at the interface of acidic aerosols. The sulfate can go on to act as a cloud seed in the atmosphere. The scientific community, therefore, seeks to understand these transformations in order to predict cloud formation and ultimately understand climate change.
For the broader public, these results have important implications for air quality. “More acidic particles can increase lung stress, so we must understand the causes of acidic aerosols,” Angle said.
The litmus test
The basics of conducting this research, like in high school chemistry labs, involved the litmus test, used to determine if a substance is acidic or basic/alkaline.
“If you dipped litmus paper in lemon juice or vinegar, it would turn red, telling you these liquids are acidic. If you dipped it in seawater, it would turn blue, because the ocean is more alkaline,” explained Angle. “When I separated sea spray aerosols based on size and applied litmus paper to them, I found that the big ones are as acidic as tomato juice and the small ones as acidic as lemon juice. This shows that the particles produced from seawater have a different pH than the original seawater.”
According to Grassian, this is an unexpected finding that adds to the understanding of sea spray aerosol, an important component of the Earth’s atmosphere.
“Oceans cover approximately 70 percent of the Earth’s surface, and sea spray aerosol is one of the most abundant aerosols by mass in the atmosphere. These measurements of nascent sea spray aerosol pH, performed in a unique ocean-atmosphere facility at Scripps Institution of Oceanography (Scripps), provide convincing data to show there is an acidification ‘across the interface,’ within minutes, when aerosols form from ocean surface waters to become airborne. We also show there is a correlation between aerosol acidity and dissolved carbon dioxide,” said Grassian, who also chairs the Department of Chemistry and Biochemistry.
SeaSCAPE opportunity
The research took place in three phases. In the first phase, Grassian and Angle worked to develop a method of using litmus paper and aerosol size-separators to measure the acidity of the highly salty particles produced from ocean waves. In the second phase, Angle worked with a large team at the CAICE during a summer experiment called SeaSCAPE in 2019. This experiment is unique because of the facilities at Scripps and the expertise within CAICE, a center directed and led by Distinguished Professor of Atmospheric Chemistry Kimberly Prather. She and her research group, including graduate students Kathryn Mayer and Jonathan Sauer, along with other CAICE investigators, were instrumental in getting the ocean-atmosphere facility running so these particular measurements could be made.

Image of a wave crashing in the hydraulics lab wave flume at Scripps Institution of Oceanography. By connecting various instruments to the flume, the CAICE team can study the same sea spray particles at the same time and understand how these particles impact our health and climate. Image created by Nigella Hillgarth.
“Using the ocean-atmosphere facility in the hydraulics laboratory at Scripps, a large glass tank with real seawater, we created and measured sea spray particles in a controlled environment,” said Angle. “Finally, during the pandemic, we performed calculations using the data that we obtained during SeaSCAPE to understand the acidification of these particles. It was a long process, but we think the payoff was worth it.”
That payoff began during earlier work in the Grassian Research Group, when Angle said he noticed that aerosols often become more acidic than the liquid used to produce them. He discussed this observation with Grassian, and they saw SeaSCAPE as an opportunity to investigate whether this acidification happens for freshly emitted sea spray particles.
“People had used litmus paper to study laboratory-generated aerosol pH before, but so far there were no successes for real world aerosols, so we saw this as a chance to push the science forward,” said Angle, adding that he is excited for the next steps of the research because it is where different pieces will come together.
“My first project in the Grassian group was measuring and controlling the acidity of individual aerosols using an instrument called an optical tweezers. With the results from SeaSCAPE, I know what the acidity of fresh sea spray aerosols should be,” said Angle. “So, I plan to use the optical tweezers to study the reactions of common pollutants in highly acidic aerosols. The results should help us better understand the chemistry of the air we breathe.”
Others from UC San Diego involved with this project include: faculty member Todd Martz; graduate students Daniel Crocker, Kathryn Mayer, Alexia Moore, Stephanie Mora Garcia, Victor Or and Jonathan Sauer; postdoctoral scholar Rebecca Simpson; CAICE Managing Director Chris Lee, undergraduate Sudarshan Srinivasan and Mahum Farhan (University of Liverpool, working at Scripps with CAICE); the University of Wisconsin-Madison’s Professor Timothy Bertram and UC Davis Professor Christopher Cappa.
This work was funded by the National Science Foundation (NSF) through CAICE (grant no. CHE-1801971).
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



