‘Molecular Distancing’ Presents Pathway to Remote Chemical Reactions
UC San Diego chemists use "fast-talking" photons to enable molecules to exchange vibrational energy
Published Date
By:
- Cynthia Dillon
Share This:
Article Content

Left to right, top: Bo Xiang, Liying Chen, Zimo Yang; middle: Joel Yuen-Zhou, Wei Xiong; bottom: Matthew Du and Raphael Ribeiro. Photos courtesy of Joel Yuen-Zhou and Wei Xiong
In this age of COVID-19, working and relating remotely is commonplace. Speedy technology provides the connection that enables it. Two UC San Diego professors of chemistry and biochemistry, Wei Xiong and Joel Yuen-Zhou, were interested in a way to improve connection between their “workers”—molecules.
In nature, the exchange of vibrational energy between molecules is painfully slow relative to the quick dissipation of energy into solvent, expressed through wasted heat. By finding a way to artificially make molecules in solution exchange vibrational energy at room temperature, Xiong and Yuen-Zhou challenge the current paradigm in chemistry. Details of their discovery are now published in Science.
“We devised a method whereby two different types of molecules can quickly communicate by exchanging photons with a so-called cavity (a setup of two mirrors that trap photons between them),” said Xiong, who led the study. “This is analogous to when two people, who cannot talk to each other when they are spatially separated, are suddenly connected via a cable. The photon in our experiment is the cable for the molecules.”
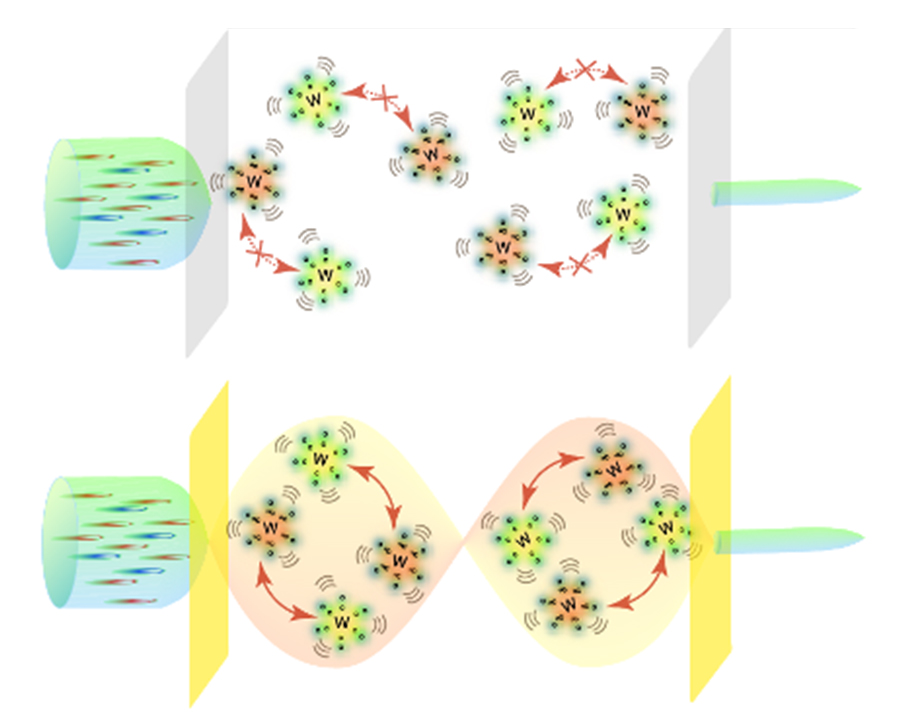
Figure. 1. Schematic illustration showing that energy transfer between vibrational modes of W(CO)6 and W(13CO)6 molecules is unfavorable in solution (top), while it is enabled by strong coupling of the molecular system to an infrared cavity mode (bottom). Credit to Bo Xiang
According to the scientists, the study introduces a generic way of engineering artificial forces between photons and molecules to enable new energy transfer pathways between molecules. Thus, many applications that were limited by poor intermolecular vibrational energy transfer become viable.
“For example, there are catalytic reactions that require energy transfer from catalysts to reactants, however, direct contact between the two would deactivate them,” explained Yuen-Zhou. “Using our method, catalysts can transfer energy to reactants without any contact, making such remote reactions happen.”
Another example is that this energy transfer mechanism enables new avenues for sensing molecules, given that each chemical species has a specific set of vibrational frequencies that altogether determine its “fingerprint.” Finally, the discovery also paves the way for the generation of exotic quantum mechanical collective states called vibrational condensates, which could be used for quantum simulation and computation.
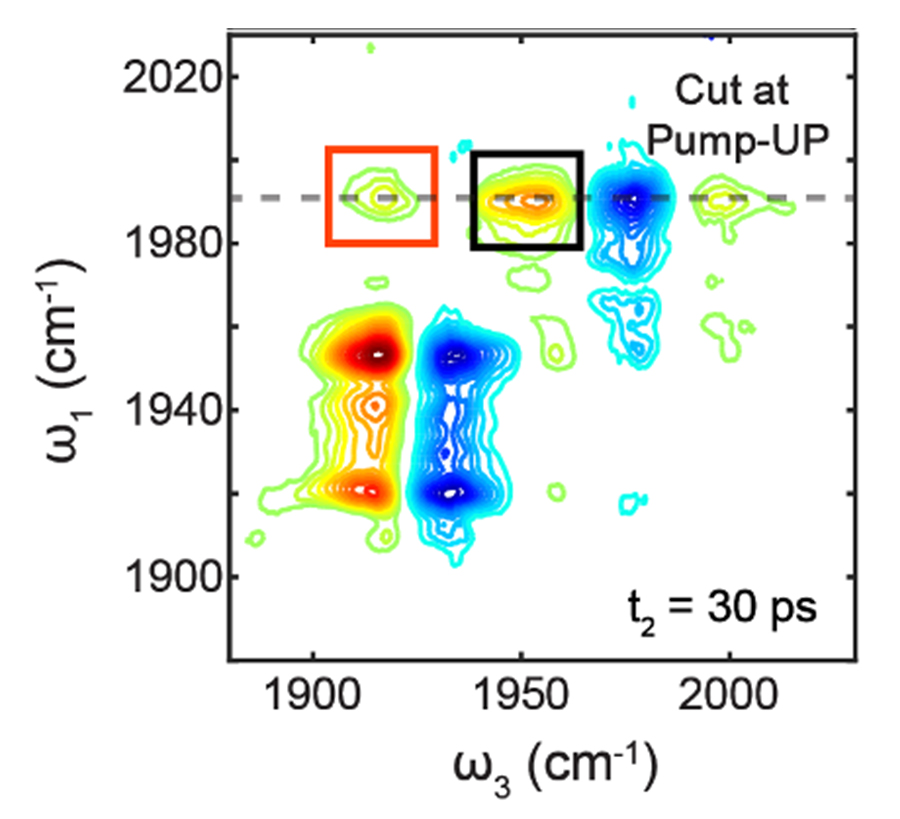
Figure 2. Evidence of vibrational energy transfer happened in solution through the aid of cavity photons. The peak labeled by the red box in 2D IR spectra indicates energy transfer between the W(CO)6 and W(13CO)6 molecules. Credit to Bo Xiang.
“At this moment, our results are of largely fundamental flavor. However, they really add new concepts into chemistry and physics on what the new rules of molecular interactions can be,” said Xiong. “We believe that they are likely to open avenues for new chemical reactions, chemical sensing and even quantum information technology platforms.”
The study was a collaborative effort between the Xiong and Yuen-Zhou labs at UC San Diego. The lead author was Bo Xiang, a UC San Diego graduate student from the Materials Science and Engineering program. Other collaborators were Raphael Ribeiro, a UC San Diego postdoctoral fellow who will be starting his independent faculty position at Emory University in the fall; graduate students Matt Du, Jiaxi Wang, Zimo Yang and Liying Chen; and Liying Chen, a UC San Diego undergraduate student at the time of the study.
Support for this study was provided by Air Force Office of Scientific Research (AFOSR) Young Investigator Program (FA9550-17-1-0094), AFOSR (award FA9550-18-1-0289) and with research instrument support by AFOSR DRUIP (FA9550-18-1-0451); the NSF CAREER Program (DMR-1848215); the U.S. Department of Energy, Office of Science, Basic Energy Sciences, CPIMS Program under Early Career Research Program award (DE-SC0019188) and the Roger Tsien Fellowship from UC San Diego Department of Chemistry and Biochemistry.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



