COVID-19, MIS-C and Kawasaki Disease Share Same Immune Response
The inflammatory disorders share similar underlying molecular patterns, a UC San Diego study reports; findings may improve disease diagnosis and treatment and support new drug targets for MIS-C
Published Date
Article Content
The emergence of COVID-19 had doctors racing to define and treat the new disease, but they soon discovered it was not the only novel illness caused by SARS-CoV-2. A subset of children infected by the virus also experienced abdominal pain, headaches, rashes and vomiting. This new set of symptoms was labeled multisystem inflammatory syndrome in children (MIS-C) and had many of its pediatric patients requiring intensive care.
As MIS-C rates rose, physicians began to note its similarities to a pre-pandemic illness, Kawasaki disease (KD), which has puzzled pediatricians for more than 50 years. MIS-C and KD share many symptoms, including fever, rash and bloodshot eyes, though KD can also lead to coronary artery aneurysms and heart attack. Unlike MIS-C, which is associated with a specific virus, KD may be triggered by a variety of infectious and environmental stimuli.
To better understand how these inflammatory syndromes compare and contrast, researchers at University of California San Diego School of Medicine collected blood and tissue samples from MIS-C and KD patients. Using artificial intelligence tools, they analyzed patterns of gene expression in both conditions and compared them to gene expression markers of COVID-19.
The study, publishing May 16, 2022 in Nature Communications, reveals that MIS-C and KD are on the same immune response continuum as COVID-19, with MIS-C being a more severe version of the response than KD. Despite these underlying similarities, the conditions do diverge in several laboratory and clinical parameters. Authors said the findings could improve disease diagnosis, monitoring and treatment in pediatric patients.
“We want our immune system to protect us from harmful stimuli, but some children are genetically predisposed to respond more intensely, leading to inflammation and unwanted symptoms across the body,” said co-corresponding author Jane C. Burns, MD, a pediatrician at Rady Children’s Hospital-San Diego and director of the Kawasaki Disease Research Center at UC San Diego School of Medicine. “The sooner we can identify and understand the child’s inflammatory condition, the better we can tailor our delivery of life-saving support.”
The research team previously identified a set of 166 genes expressed in viral respiratory diseases, including COVID-19, a subset of which also corresponded to disease severity. Researchers found that this same “gene signature” also applied to both MIS-C and KD, suggesting the conditions all stem from a similar underlying mechanism, which involves the rapid release of IL15/IL15RA cytokines.
The team then looked at a separate set of 13 genes used to identify KD, and found that a computer program trained to look for this genetic signature could not tell the KD and MIS-C samples apart.
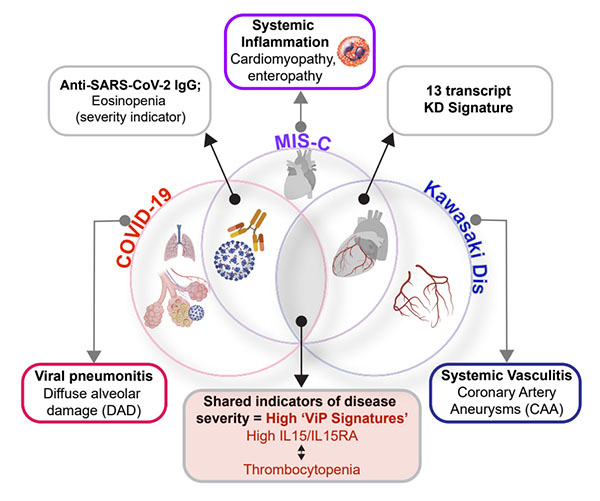
UC San Diego researchers summarize the similarities and differences between COVID-19, MIS-C and Kawasaki disease, three conditions unified by the same immune-associated gene signature.
“We were not expecting that,” said co-corresponding author Pradipta Ghosh, MD, professor of medicine and cellular and molecular medicine at UC San Diego School of Medicine. “We analyzed MIS-C and KD through the lens of two distinct gene signatures, and both experiments told us these diseases are closely related.”
Ghosh said the two gene signatures likely represent different parts of the same broader immune response.
While the study provides a new unifying framework for these diseases, it also identifies a few subtle differences. For example, MIS-C patients had lower blood platelet and eosinophil counts, two features that can be measured from routine blood tests. And, while many serum cytokines were similarly elevated in both conditions, a select few were more elevated in MIS-C than in KD samples.
Authors noted that therapeutics targeting some of these cytokines, including TNFα and IL1β, have already been approved by the U.S. Food and Drug Administration (FDA) and are being tested as novel treatments for MIS-C.
“We believe our findings have a high potential to impact clinical trial planning immediately, and also shape clinical guidelines and patient care down the line,” said co-corresponding author Debashis Sahoo, PhD, associate professor of pediatrics and computer science at UC San Diego School of Medicine and UC San Diego Jacobs School of Engineering.
Co-authors include: Gajanan D. Katkar, Chisato Shimizu, Jihoon Kim, Soni Khandelwal, Adriana H. Tremoulet, John T. Kanegaye, Pediatric Emergency Medicine Kawasaki Disease Research Group and Soumita Das, all at UC San Diego, as well as Joseph Bocchini of the Willis-Knighton Health System.
This work was funded, in part, by the National Institutes of Health (grants R01-GM138385, R01- 854 AI141630, R01DK107585 and R01-AI155696), UCOP-RGPO (grants R01RG3780, R00RG2628 and R00RG2642), iDASH (grants U54HL108460 and R01HL140898), the Patient Outcomes Research Institute, the Gordon and Marilyn Macklin Foundation, the American Association of Immunologists Intersect Fellowship Program for Computational Scientists and Immunologists, and a UC San Diego Stem Cell Center Pilot award.
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.