Chasing a Moving Target: Research Ethics in a Digital Age
Story by:
Published Date
Story by:
Topics covered:
Share This:
Article Content
On a Monday in March, groups of four or five people — students, researchers, staff members from across the UC San Diego campus — huddled intently together over thick printouts of privacy policies. Their challenge? Not only to wade through dense paragraphs of legalese, but also to reimagine how to present the information in a relevant, engaging and meaningful way.
The half-day Privacy Policy Design-a-thon, hosted by UC San Diego ethicist and researcher Camille Nebeker in UC San Diego Qualcomm Institute’s (QI) Atkinson Hall, aimed to harness participants’ insights to take a fresh look at privacy policies, a little-examined aspect of research consent, to empower users to make truly informed decisions.
As an example, Nebeker noted that many digital health studies use third-party technology like Fitbit sensors, which can track an individual’s exact geographic location down to movements through the rooms of a house or stops at a local bar. Researchers, Institutional Review Board (IRB) members and study participants may not be aware of the scope of the data collected.
“Privacy policies are not meant to be read or understood,” said Nebeker, professor in the UC San Diego Herbert Wertheim School of Public Health and Human Longevity Science and an affiliate of both UC San Diego’s Design Lab and QI. “Regardless, [as researchers,] we have a responsibility to know how data are collected, stored and used by third-party commercial vendors who supply products used in digital health research. We can think outside the box and find a respectful way to communicate to a prospective participant or a patient. We can do better, and that’s the whole purpose of this workshop.”

As the workshop progressed, participants became more animated as they debated the key problems, brainstormed best approaches, collaborated with other groups, and sketched out new privacy policy prototypes. At the end of the session, teams presented fresh approaches that ranged from interactive apps to executive summaries, video presentations and gamified designs.
“The workshop was exactly what I’d hoped,” said Nebeker, who had spent three months planning the event. “Now we’ll try to unpack the pieces of the prototypes, identify unique concepts and send out a survey to ask for input from more people.”
In the work, supported by the Patient-Centered Outcomes Research Institute, Nebeker and her team will also examine if artificial intelligence, specifically large language models, could be useful in helping to convey key privacy policy information. Their goals include benchmarking AI tools for accuracy and evaluating AI-generated output.
Data, Data Everywhere
The issues tackled in the Privacy Policy Design-a-thon fit squarely into Nebeker’s larger scientific and professional interests.
“My interest is in digital health,” she said. “What do researchers need to think about as they’re selecting tools to gather participant data in the real world? How do researchers make decisions? How do they convey elements of data management to the IRB? What do participants need to know before making a decision about participating in a study?”

In 2017, Nebeker made a successful case to the vice chancellor of the UC San Diego Office of Research and Innovation for seed funding to launch the Research Center of Optimal Digital Ethics (ReCODE Health) to examine these types of questions. Nebeker noted that few people at the time were looking at how to shape ethical practices in an era where the volume of data was exploding — a situation that has only accelerated.
“Our data are flowing everywhere, all the time,” she said. “In the last 15 to 20 years research has gone from addressing pretty straightforward questions to really wicked problems in an era of digital technologies, AI and machine learning.”
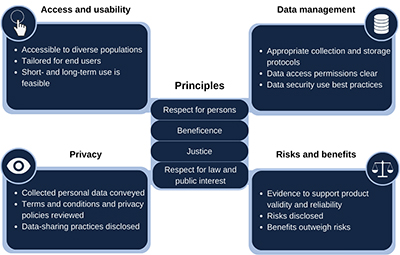
Initially, the group at ReCODE Health focused on understanding who was involved in the digital health research ecosystem and identifying novel ethical challenges that were arising. These efforts led to creating resources, including a decision-support tool for researchers called the Digital Health Checklist, which was anchored by accepted ethical principles and draws attention to issues of access and usability, risks and benefits, privacy and data management.
A Practical and Supportive Approach
Nebeker and team are now in the process of evolving the digital health checklist into a more robust risk-management tool. The upgraded instrument would help researchers not only identify risks, but also mitigate them and assess them across different individuals.
“What’s ‘low risk’ for one person might not be low risk for somebody who is not documented or who is not digitally literate,” Nebeker noted.
The team at ReCODE Health has also been exploring how to communicate complex concepts to research participants. One of the team’s recent studies, published in the Journal of the American Medical Informatics Association (2025), found dataflow diagrams — visual tools that illustrate how participant data moves through a study — could enhance informed consent communication.
Another study, published in the Journal of Medical Internet Research (2025), used a survey to find what people value most when reviewing informed consent documents. Priorities included clear explanations of data use, control over information and opportunities to revisit consent decisions.
Supporters of ReCODE Health’s work have included the Robert Wood Johnson Foundation, IBM, the National Science Foundation (NSF) and the National Institutes of Health (NIH).
Commitment to Teaching and Learning
In addition to her roles as scientist and ReCODE Health director, Nebeker heads the UC San Diego Research Ethics Program, a position she has held for the last three years.
“As director, I’m responsible for educating our campus about any form of research ethics, whether it’s designing, conducting or reporting studies — all things regulated and unregulated,” she said.
She noted the Scientific Ethics course, conducted by faculty affiliated with the UC San Diego Research Ethics Program, is designed to teach participants how to recognize ethical issues, ask questions and find resources to help manage any issues that come up. Close to 400 people participate in the training every year.
“While our courses and workshops are used to comply with NIH and NSF Responsible Conduct of Research training requirements, it’s not just jumping through a compliance hoop,” Nebeker said. “There’s a real commitment to teaching and learning and developing the skills needed to navigate a complex research environment.”
One of the perks of her involvement in the Research Ethics Program has been an office in Atkinson Hall, where she can benefit from QI’s interdisciplinary, technology-forward environment.
“[Atkinson Hall] is such a sweet spot for this work,” she said. “I am in public health, and I could work two doors down from somebody in robotics, and across the hall from somebody in economics. It’s fascinating how we intersect.”
In fact, her proximity to researchers in other disciplines has already led directly to her involvement in a UC San Diego study of older adults in which sensors gather data to study healthy aging.
All the while, Nebeker remains focused on the future. “My interest is really in how we shape the research that’s coming — that is here, but that’s also evolving very rapidly,” Nebeker said, “so we can stay current with the change of pace, the change of scope and the change of risk.”
Learn more at the ReCODE Health, UC San Diego Research Ethics Program and UC San Diego Qualcomm Institute websites.
Learn more about research and education at UC San Diego in: Artificial Intelligence
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.




