Biochemists make ‘Elbow Room’ for Nanostructures with new Toolkit
Combining DNA and RNA, UC San Diego researchers cut a path to the forefront of nanotechnology
Published Date
By:
- Cynthia Dillon
Share This:
Article Content
If you’ve ever put together a jigsaw puzzle or built with Legos, you might understand the importance of the corner piece—it helps assemble the puzzle and link sets of blocks. Elbows and knees function similarly within the human body. Biochemically, University of California San Diego researchers created something like it, too—an architectural joint made from nucleic acid. Now imagine a puzzle or a set of Legos that assembles a complex structure, by itself, with only a shake of the box.
Combining the biomolecules DNA and RNA, UC San Diego’s Thomas Hermann and his graduate students Alba Monferrer and Douglas Zhang created robust modules that facilitate the self-assembly of polygonal nanoshapes—really tiny triangles, squares, pentagons and hexagons measured at the nanometer scale. Their findings reported in a recent issue of Nature Communications could apply to the manufacture of self-assembling nanomaterials and to the creation of environmentally responsive sensors. This could lead to new methods for making nanoscale devices and more economically efficient, medical, point-of-care diagnostics for rapid bedside testing.
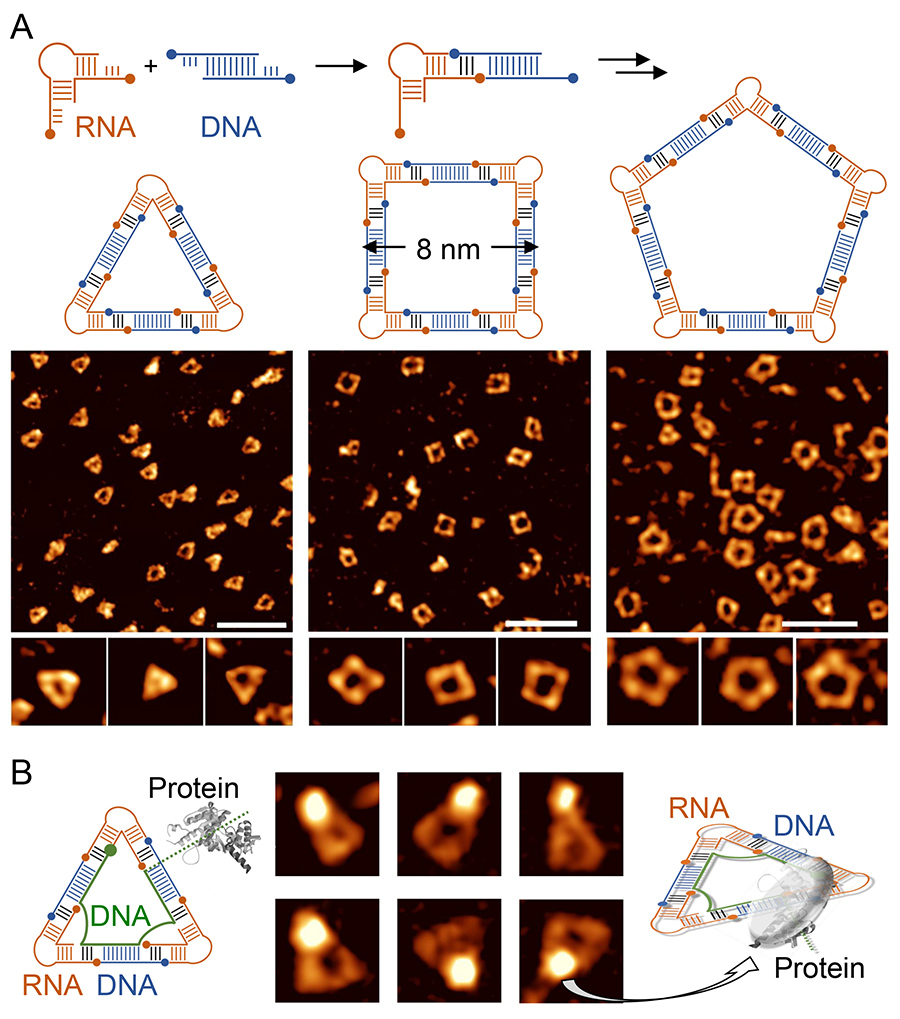
Figure: Self-assembly and imaging of RNA-DNA hybrid nanoshapes. (A) Architectural joints of RNA and DNA modules self-assemble to form triangles, squares or pentagons with side lengths of 8 nanometers, as shown here for example. Atomic force microscopy (AFM), an imaging technique that shows details of molecular assemblies at a resolution more than 1000 higher than an optical microscope, has been used to capture snapshots of the nanoshapes. (B) The nucleic acid building blocks of the nanoshapes can be designed to carry protein binding sites (green DNA strand) which allow precise positioning of proteins on the nanoshapes. In the AFM images, the protein is visible as a bright spot associated with one corner of the RNA-DNA triangle. AFM imaging for this research was performed by Alexander Lushnikov at the University of Nebraska Medical Center at Omaha. Figure by Thomas Hermann
“The two types of nucleic acid have not been used in combination to enhance structural diversity or for partitioning of functional and architectural roles,” explained Hermann, professor of chemistry and biochemistry and associate dean in the Division of Physical Sciences at UC San Diego. “It excites us to create self-assembling materials from biological molecules, which we use to precisely position features that are on the scale of a nanometer.”
To visualize a nanometer, picture a sheet of paper, which is approximately 100,000 nanometers thick … or a strand of hair from your head, which is between 80,000 and 100,000 nanometers wide … or consider that there are more than 25,400,000 nanometers in one inch. Really tiny.
As another point of reference, current smartphones operate with silicon chips that contain electronic circuits measuring just above 10 nanometers. Achieving sub-10, nanometer-sized nanostructures would mark a scientific breakthrough, and the findings of Hermann’s group are within that scope. The work by Hermann and his research colleagues essentially offers a toolkit for the systematic development of programmable nanoshape platforms that could be applied to molecular recognition, sensor and catalyst development, and protein interaction studies. The ability to program interactions between nucleic acid modules through base-pairing of DNA and RNA is foundational to the researchers’ approach of additive manufacturing for nanoscale materials.
Hermann noted that DNA serves as a blueprint, a single copy of a valuable map instructing all processes in every cell. Copies of it are made by RNA, which of the two biomolecules is more labile—ephemeral—and, in addition, can engage and adopt a diverse range of structures relative to the stability DNA provides.
“If you think of Legos, RNA is the distinguishing technical piece,” said Hermann. “The modular blueprint of the RNA-DNA hybrid structures enables extensive, self-assembling nanoshapes through design parameters that are amenable to independent optimization.”
Using soft materials like DNA and RNA for nanotechnology is cutting-edge science. In fact, Hermann recently returned from organizing and chairing the 2019 Gordon Research Conference (GRC) on RNA Nanotechnology—only the third meeting in a biennial series covering the timely topic since 2015. The conference promotes scientific exchange and interdisciplinary collaboration among researchers working in diverse fields, ranging from chemistry and biophysics of nucleic acids to biology, medicine and materials sciences.
“Gordon Research Conferences are among the most prestigious scientific conferences and provide an international forum for frontier research in the biological and physical sciences,” explained Hermann. “It has been a great honor to help put RNA nanotechnology on the map by serving as a chair during the inaugural years of this GRC, and by presenting leading-edge research conducted at UC San Diego at a venue that brings together the foremost experts in the field.”
This study was supported by the National Science Foundation, Chemical Measurement & Imaging Program (grant CHE CMI 16082870 to Thomas Hermann); a Catalan Fundació Joan Riera i Gubau Fellowship to Alba Monferrer and a pre-doctoral fellowship to Douglas Zhang by the UC San Diego Molecular Biophysics Training Grant (NIH T32 GM008326).
Share This:
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



