AI Helps Unravel a Cause of Alzheimer’s Disease and Identify a Therapeutic Candidate
Story by:
Published Date
Story by:
Topics covered:
Share This:
Article Content
A new study found that a gene recently recognized as a biomarker for Alzheimer’s disease is actually a cause of it, due to its previously unknown secondary function. Researchers at the University of California San Diego used artificial intelligence to help both unravel this mystery of Alzheimer’s disease and discover a potential treatment that obstructs the gene’s moonlighting role.
The research team published their results on April 23 in the journal Cell.
About one in nine people aged 65 and older has Alzheimer’s disease, the most common cause of dementia. While some particular genes, when mutated, can lead to Alzheimer’s, that connection only accounts for a small percentage of all Alzheimer’s patients. The vast majority of patients do not have a mutation in a known disease-causing gene; instead, they have “spontaneous” Alzheimer’s, and the causes for that are unclear.
Discovering those causes could ultimately improve medical care.
“Unfortunately, treatment options for Alzheimer’s disease are very limited. And treatment responses are not outstanding at this moment,” said study senior author Sheng Zhong, a professor in the Shu Chien-Gene Lay Department of Bioengineering at the UC San Diego Jacobs School of Engineering.
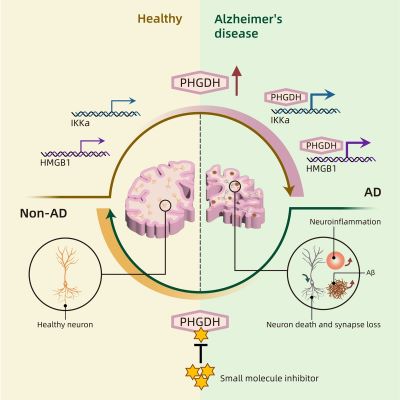
So Zhong and his team took a closer look at phosphoglycerate dehydrogenase (PHGDH), which they had previously discovered as a potential blood biomarker for early detection of Alzheimer’s disease. In a follow-up study, they later found that expression levels of the PHGDH gene directly correlated with changes in the brain in Alzheimer’s disease; in other words, the higher the levels of protein and RNA produced by the PHGDH gene, the more advanced the disease. That correlation has since been verified in multiple cohorts from different medical centers, according to Zhong.
Intrigued by this reproducible correlation, the research team decided to investigate in this latest study whether there was a causal effect. Using mice and human brain organoids, the researchers found that altering the amounts of PHGDH expression had consequential effects on Alzheimer’s disease: lower levels corresponded to less disease progression, whereas increasing the levels led to more disease advancement. Thus, the researchers established that PHGDH is indeed a causal gene to spontaneous Alzheimer’s disease.
In further support of that finding, the researchers determined—with the help of AI—that PHGDH plays a previously undiscovered role: it triggers a pathway that disrupts how cells in the brain turn genes on and off. And such a disturbance can cause issues, like the development of Alzheimer’s disease.
Moonlighting role
PHGDH creates an enzyme key for the production of serine, an essential amino acid and a neurotransmitter. Because PHGDH’s enzymatic activity was its only known role, the researchers hypothesized that its metabolic function must be connected to an Alzheimer’s outcome. However, all their experiments designed to prove so failed.
“At that time, our study hit a wall, and we didn’t have a clue of what mechanism it is,” said Zhong.
But another Alzheimer’s project in his lab, which did not focus on PHGDH, changed all this. A year ago, that project revealed a hallmark of Alzheimer’s disease: a widespread imbalance in the brain in the process where cells control which genes are turned on and off to carry out their specific roles.
The researchers were curious if PHGDH had an unknown regulatory role in that process, and they turned to modern AI for help.
With AI, they could visualize the three-dimensional structure of the PHGDH protein. Within that structure, they discovered that the protein has a substructure that is very similar to a known DNA-binding domain in a class of known transcription factors. The similarity is solely in the structure and not in the protein sequence.>
Zhong said, “It really demanded modern AI to formulate the three-dimensional structure very precisely to make this discovery.”
After discovering the substructure, the team then demonstrated that with it, the protein can activate two critical target genes. That throws off the delicate balance, leading to several problems and eventually the early stages of Alzheimer’s disease. In other words, PHGDH has a previously unknown role, independent of its enzymatic function, that through a novel pathway leads to spontaneous Alzheimer’s disease.
That ties back to the team’s earlier studies: the PHGDH gene produced more proteins in the brains of Alzheimer’s patients compared to the control brains, and those increased amounts of the protein in the brain triggered the imbalance. While everyone has the PHGDH gene, the difference comes down to the expression level of the gene, or how many proteins are made by it.
Treatment option
Now that the researchers uncovered the mechanism, they wanted to figure out how to intervene and thus possibly identify a therapeutic candidate, which could help target the disease.
While many current treatments focus on treating the abnormal buildup of the sticky protein called beta-amyloid in the brain, some studies suggest that treating those plaques may be ineffective: essentially by that stage of accumulation, treatment is too late. But the critical pathway discovered in this study is upstream, so preventing this pathway can reduce amyloid plaque formation in the first place.
Given that PHGDH is such an important enzyme, there are past studies on its possible inhibitors. One small molecule, known as NCT-503, stood out to the researchers because it is not quite effective at impeding PHGDH’s enzymatic activity (the production of serine), which they did not want to change. NCT-503 is also able to penetrate the blood-brain-barrier, which is a desirable characteristic.
They turned to AI again for three-dimensional visualization and modeling. They found that NCT-503 can access that DNA-binding substructure of PHGDH, thanks to a binding pocket. With more testing, they saw that NCT-503 does indeed inhibit PHGDH’s regulatory role.
When the researchers tested NCT-503 in two mouse models of Alzheimer’s disease, they saw that it significantly alleviated Alzheimer’s progression. The treated mice demonstrated substantial improvement in their memory and anxiety tests. These tests were chosen because Alzheimer’s patients suffer from cognitive decline and increased anxiety.
The researchers do acknowledge limitations of their study. One being that there is no perfect animal model for spontaneous Alzheimer’s disease. They could test NCT-503 only in the mouse models that are available, which are those with mutations in those known disease-causing genes.
Still, the results are promising, according to Zhong.
“Now there is a therapeutic candidate with demonstrated efficacy that has the potential of being further developed into clinical tests,” said Zhong. “There may be entirely new classes of small molecules that can potentially be leveraged for development into future therapeutics.”
An advantage of small molecules is that they could even be administered orally, he added, unlike the current treatments that require infusions.
The next steps will be to optimize the compound and subject it to FDA IND-enabling studies.
Paper: “Transcriptional regulation by PHGDH drives amyloid pathology in Alzheimer’s disease.” Co-authors include Junchen Chen*, Fatemeh Hadi*, Xingzhao Wen, Wenxin Zhao, Ming Xu, Shuanghong Xue, Pei Lin, Riccardo Calandrelli, John Lalith Charles Richard, Zhixuan Song, Jessica Li, Alborz Amani, Yang Liu and Xu Chen, all of UC San Diego.
*These authors contributed equally
This work is partially funded by the National Institutes of Health (grants R01GM138852, DP1DK126138, UH3CA256960, R01HD107206, R01AG074273 and R01AG078185).
Disclosure: Sheng Zhong is a founder and shareholder of Genemo, Inc. and Neurospan, LLC. The remaining authors declare no competing interests.
Learn more about research and education at UC San Diego in: Artificial Intelligence
The study co-authors (from left to right) Sheng Zhong, Junchen Chen, Wenxin Zhao, Ming Xu, Shuanghong Xue, Zhixuan Song and Fatemeh Hadi uncovered that the PHGDH gene can trigger a pathway that disrupts how cells in the brain turn genes on and off, which can cause diseases like Alzheimer’s to develop. Photo credit: Zhong lab
You May Also Like
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.