A Giant Leap Forward in Wireless Ultrasound Monitoring for Subjects in Motion
Engineers at UC San Diego develop a fully integrated system for deep-tissue monitoring
Published Date
Story by:
Media contact:
Share This:
Article Content
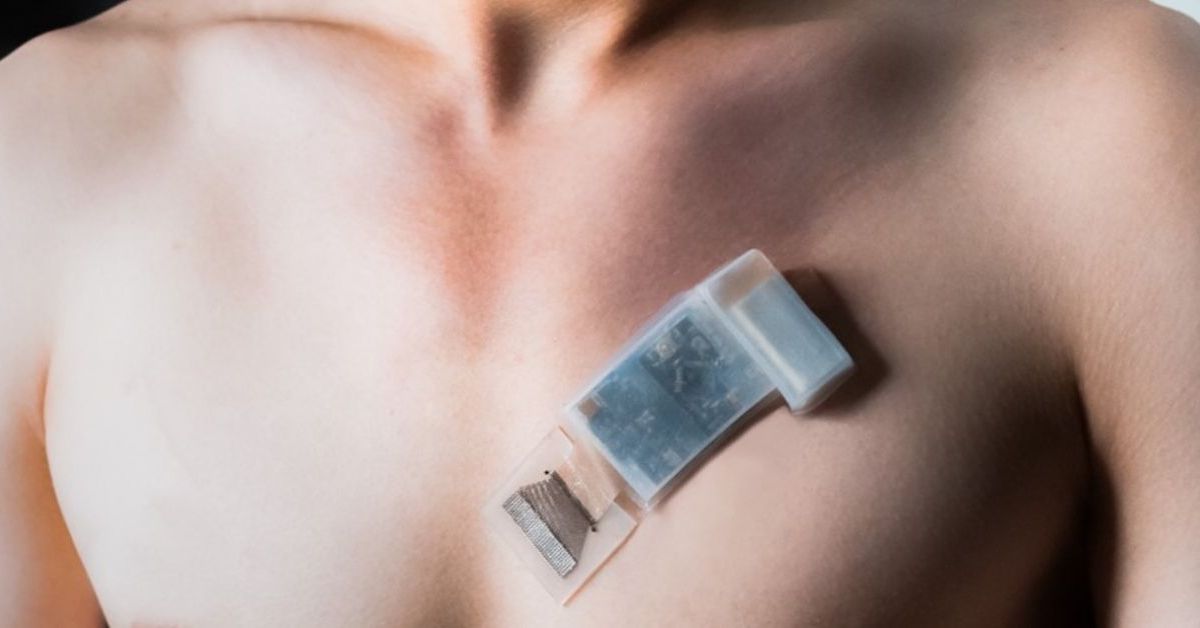
A team of engineers at the University of California San Diego has developed the first fully integrated wearable ultrasound system for deep-tissue monitoring, including for subjects on the go. It facilitates potentially life-saving cardiovascular monitoring and marks a major breakthrough for one of the world’s leading wearable ultrasound labs. The paper, “A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects,” is published in the May 22, 2023 issue of Nature Biotechnology.
“This project gives a complete solution to wearable ultrasound technology—not only the wearable sensor, but also the control electronics are made in wearable form factors,” said Muyang Lin, a Ph.D. candidate in the Department of Nanoengineering at UC San Diego and the first author on the study. “We made a truly wearable device that can sense deep tissue vital signs wirelessly.”

A wearable ultrasonic-system-on-patch for deep tissue monitoring. Photo by Muyang Lin for the Jacobs School of Engineering at UC San Diego. Full-size gallery.
The research emerges from the lab of Sheng Xu, a professor of nanoengineering at UC San Diego Jacobs School of Engineering and corresponding author of the study.
This fully integrated autonomous wearable ultrasonic system-on-patch (USoP) builds on the lab’s previous work in soft ultrasonic sensor design. However, previous soft ultrasonic sensors all require tethering cables for data and power transmission, which largely constrains the user’s mobility. In this work, it includes a small, flexible control circuit that communicates with an ultrasound transducer array to collect and transmit data wirelessly. A machine learning component helps interpret the data and track subjects in motion.
According to the lab’s findings, the ultrasonic system-on-patch allows continuous tracking of physiological signals from tissues as deep as 164 mm, continuously measuring central blood pressure, heart rate, cardiac output, and other physiological signals for up to twelve hours at a time.
“This technology has lots of potential to save and improve lives,” Lin said. “The sensor can evaluate cardiovascular function in motion. Abnormal values of blood pressure and cardiac output, at rest or during exercise, are hallmarks of heart failure. For healthy populations, our device can measure cardiovascular responses to exercise in real time and thus provide insights into the actual workout intensity exerted by each person, which can guide the formulation of personalized training plans.”
The ultrasonic system-on-patch also represents a breakthrough in the development of the Internet of Medical Things (IoMT), a term for a network of medical devices connected to the internet, wirelessly transmitting physiological signals into the cloud for computing, analysis and professional diagnosis.
Thanks to technological advances and the hard work of clinicians over the last few decades, ultrasound has received an ongoing wave of interest, and the Xu lab is often mentioned in the first breath as an early and enduring leader in the field, particularly in wearable ultrasound. The lab took devices that were stationary and portable and made them stretchable and wearable, driving a transformation across the landscape of healthcare monitoring. Its strength rests in part on its close collaboration with clinicians. “Although we are engineers, we do know the medical problems that clinicians face,” Lin said. “We have a close relationship with our clinical collaborators and always get valuable feedback from them. This new wearable ultrasound technology is a unique solution to address many vital sign monitoring challenges in clinical practice.”
While developing its latest innovation, the team was surprised to discover that it had more capabilities than initially anticipated.
“At the very beginning of this project, we aimed to build a wireless blood pressure sensor,” said Lin. “Later on, as we were making the circuit, designing the algorithm and collecting clinical insights, we figured that this system could measure many more critical physiological parameters than blood pressure, such as cardiac output, arterial stiffness, expiratory volume and more, all of which are essential parameters for daily health care or in-hospital monitoring.”
Moreover, when the subject is in motion, there will be relative movement between the wearable ultrasonic sensor and the tissue target, which will require frequent manual readjustment of the wearable ultrasonic sensor to keep track of the moving target. In this work, the team developed a machine learning algorithm to automatically analyze the received signals and choose the most appropriate channel to keep track of the moving target.
However, when the algorithm is trained using one subject’s data, that learning may not be transferable to other subjects, making the results inconsistent and unreliable.
“We eventually made the machine learning model generalization work by applying an advanced adaptation algorithm,” said Ziyang Zhang, a master’s student in the Department of Computer Science and Engineering at UC San Diego and co-first author on the paper. “This algorithm can automatically minimize the domain distribution discrepancies between different subjects, which means the machine intelligence can be transferred from subject to subject. We can train the algorithm on one subject and apply it to many other new subjects with minimal retraining.”
Moving forward, the sensor will be tested among larger populations. “So far, wehave only validated the device performance on a small but diverse population,” said Xiaoxiang Gao, a postdoctoral scholar in the Department of Nanoengineering at UC San Diego and co-first author on the study. “As we envision this device as the next generation of deep-tissue monitoring devices, clinical trials are our next step.”
Xu is the co-founder of Softsonics, LLC, which plans to commercialize the technology.
Paper: “A fully integrated wearable ultrasound system to monitor deep tissues in moving subjects.” Coauthors include Muyang Lin*, Department of NanoEngineering, UC San Diego; Ziyang Zhang*, Department of Computer Science and Engineering, UC San Diego; Xiaoxiang Gao*, Yizhou Bian, Ray S. Wu, Geonho Park, and Zhiyuan Lou, Department of NanoEngineering, UC San Diego; Zhuorui Zhang, Department of Mechanical Engineering, Massachusetts Institute of Technology; Xiangchen Xu, Department of NanoEngineering, UC San Diego; Xiangjun Chen, Materials Science and Engineering Program, UC San Diego; Andrea Kang, Department of Electrical and Computer Engineering, UC San Diego; Xinyi Yang, Materials Science and Engineering Program, UC San Diego; Wentong Yu and Lu Yin, Department of NanoEngineering, UC San Diego; Chonghe Wang, Department of Mechanical Engineering, Massachusetts Institute of Technology; Baiyan Qi and Sai Zhou, Materials Science and Engineering Program, UC San Diego; Hongjie Hu and Hao Huang, Department of NanoEngineering, UC San Diego; Mohan Li, Department of Electrical and Computer Engineering, UC San Diego; Yue Gu, Materials Science and Engineering Program, UC San Diego, and Department of Neurosurgery, Yale University; Jing Mu, Materials Science and Engineering Program, UC San Diego; Albert Yang, Department of Bioengineering, UC San Diego; Amer Yaghi and Yimu Chen, Department of NanoEngineering, UC San Diego; Yusheng Lei, Department of NanoEngineering, UC San Diego, and Department of Chemical Engineering, Stanford University; Chengchangfeng Lu, Department of Electrical and Computer Engineering, UC San Diego; Ruotao Wang and Joseph Wang, Department of NanoEngineering, UC San Diego; Shu Xiang, Softsonics LLC, San Diego; Erik B. Kistler, Department of Bioengineering and Department of Anesthesiology and Critical Care, UC San Diego; Nuno Vasconcelos, Department of Electrical and Computer Engineering, UC San Diego; and Sheng Xu**, Department of Nanoengineering, Department of Electrical and Computer Engineering, Materials Science and Engineering Program and Department of Bioengineering, UC San Diego; Department of Radiology, School of Medicine, UC San Diego; and Softsonics, Inc.
*These authors contributed equally.
**Corresponding author
This research was partially supported by the Air Force Research Laboratory (AFRL) under agreement number FA8650-18-2-5402 and National Institutes of Health (NIH) (grant no. 1 R01 EB033464-01).
Share This:
Stay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.