UC San Diego Biologists Discover Genes That Repair Nerves After Injury
By:
- Kim McDonald
Published Date
By:
- Kim McDonald
Share This:
Article Content
Biologists at the University of California, San Diego have identified more than 70 genes that play a role in regenerating nerves after injury, providing biomedical researchers with a valuable set of genetic leads for use in developing therapies to repair spinal cord injuries and other common kinds of nerve damage such as stroke.
In the September 22 issue of the journal Neuron, the scientists detail their discoveries after an exhaustive two-year investigation of 654 genes suspected to be involved in regulating the growth of axons—the thread-like extensions of nerve cells that transmit electrical impulses to other nerve cells. From their large-scale genetic screen, the researchers identified 70 genes that promote axon growth after injury and six more genes that repress the re-growth of axons.
“We don’t know much about how axons re-grow after they’re damaged,” said Andrew Chisholm, a professor of biology at UC San Diego. “When you have an injury to your spinal cord or you have a stroke you cause a lot of damage to your axons. And in your brain or spinal cord, regeneration is very inefficient. That’s why spinal cord injuries are basically untreatable.”
Chisholm and UC San Diego biology professor and HHMI Investigator Yishi Jin headed the collaborative research team, which also included researchers from the University of Oregon.
While scientists in recent decades have gained a good understanding of how nerve cells, or neurons, develop their connections in the developing embryo, much less is known about how adult animals and humans repair—or fail to repair—those connections when axons are damaged.
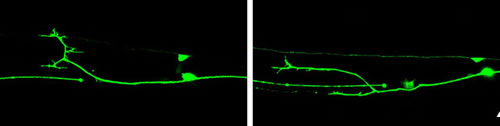
Regrowing axons 12 hours (left) and 24 hours (right) after injury. Credit: Lizhen Chen, UC San Diego
“There are many processes not involved in early development that are involved in switching the neurons to this re-growth mode,” said Chisholm. “In essence what we found are genes that people had not suspected previously to be part of this process.”
Of particular interest to the UC San Diego biologists are the six genes that appear to repress the growth of axons.
“The discovery of these inhibitors is probably the most exciting finding,” said Chisholm, because identifying and eliminating the inhibiting factors to the re-growth of axons could be just as essential as the biochemical pathways that promote axon re-growth in repairing spinal cord injuries and other kinds of nerve damage.
The scientists were also surprised to learn that some of the genes they found to be involved in the re-growth of axons were known to have other functions, such as regulating the release of neurotransmitters.
“This was in large part unexpected,” said Chisholm. “These genes had not been implicated in the re-growth of axons before.”
To find the 76 genes, the researchers conducted painstaking experiments on more than 10,000 tiny laboratory roundworms known as C. elegans. The first step involved developing genetic mutants of these transparent roundworms for each one of 654 genes that were suspected to play a role in the regulation of axon regrowth in worms, fruit flies and mice. They then labeled the roundworm neurons with green fluorescent protein and, with a precise surgical laser, damaged a specific axon.
“The goal was to study this process in its simplest form,” said Chisholm. “Because the animals are essentially transparent, we can see the axons expressing this green fluorescent protein.”
By examining the re-growth, or lack of growth, of the damaged axon 24 hours later, the scientists were then able to determine which of these 654 genes were actually important to axon re-growth.
Chisholm said that while the 76 genes identified are believed to have similar roles in mammals as well as roundworms, because their functions were “conserved” by the organisms through evolution, he and his research team are now collaborating with other investigators to conduct experiments on mice to verify this connection and determine which of these genes are the most critically important.
“Worms are clearly different from mammals,” he added. “But there will be a core of conserved molecules doing the same job.”
In addition to Chisholm and Jin, the UC San Diego biologists involved in the study were Lizhen Chen, Zhiping Wang, Anindya Ghosh-Roy, Thomas Hubert, Dong Yan, and Zilu Wu. Sean O’Rourke and Bruce Bowerman from the University of Oregon were also part of the team.
The research project was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute.
Share This:
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.



