$12.7M Grant to Establish New Center to Investigate Genetic Basis of Autism and Schizophrenia
Funded by a California Institute for Regenerative Medicine grant, researchers in the UC San Diego Verge Center aim to uncover the genetic mechanisms behind neuropsychiatric disorders
Story by:
Published Date
Article Content
Neuropsychiatric disorders—including schizophrenia and autism—are complex conditions that have been linked to many genes involved in early brain development. However, the neurobiological mechanisms through which these genes influence brain function and mental health remain poorly understood.
To address this, an interdisciplinary team of scientists and clinicians led by Jonathan Sebat, a professor in the Departments of Psychiatry and Cellular & Molecular Medicine at UC San Diego School of Medicine, has received a $12.7 million grant from the California Institute for Regenerative Medicine (CIRM). With this funding, they will establish the UC San Diego Verge Center: Convergence and Divergence of Genes on Neurodevelopment and Mental Health, one of five new centers supported through CIRM’s pilot ReMIND (Research Using Multidisciplinary, Innovative Approaches in Neuro Diseases) program.

The new UC San Diego Verge Center will take a data-driven approach to defining the mechanisms underlying psychiatric traits. Researchers will use cutting-edge stem cell and brain organoid technologies to investigate how specific genetic mutations influence brain development.
This approach represents a departure from traditional studies that focus on one gene at a time: with laboratory studies comparing a mutant form of the gene to its typical form and clinical studies comparing people that have a gene mutation to people that are “neurotypical.”
“These previous studies have been fascinating and complex,” says Sebat. “Genes associated with autism have been found to have a wide range of effects on brain development and on cognitive function, but when we compare one autism gene to the next, it's difficult to distinguish what are the common pathways and neurodevelopmental mechanisms."
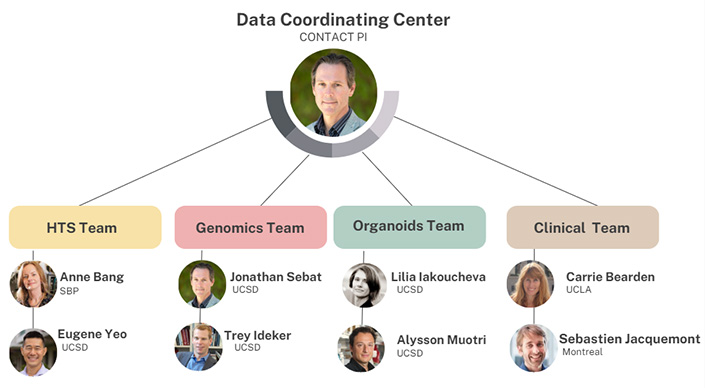

The Verge Center will take a data-driven approach to characterize the functions of genes in the developing brain by integrating large datasets on the effects of gene mutations on neurodevelopment collected in the laboratory and in the clinic.
Uniting research teams in genetics, neuroscience, and clinical psychiatry from UC San Diego and partner institutions, the center will collaborate on four major projects that build on strong research programs in the genetics of psychiatric disorders.

One of these efforts is led by Lilia Iakoucheva, a professor in the Department of Psychiatry, and Alysson Muotri, a professor in the Departments of Pediatrics and Cellular & Molecular Medicine and Moores Cancer Center Member.
Their teams will study how genetic risk factors for autism and schizophrenia impact brain development using brain organoids—three-dimensional cell culture models that mimic human brain structure and function.

These models allow the researchers to observe brain development at the cellular level, providing insight into how genetic changes affect brain wiring and structure. The findings will be compared to patient brain scans and activity measurements from patients to better understand how these mutations manifest in the human brain.
Another key project, led by Gene Yeo, a professor in the Department of Cellular & Molecular Medicine and Moores Cancer Center Member, and Anne Bang, director of cell biology at the Prebys Center at Sanford Burnham Prebys Medical Discovery Institute, will use high-throughput screening platforms to investigate the functions >100 genes in brain cells. They will study how loss of function or gain of function of genes affect neurons grown from stem cells in the lab.

The clinical teams, led by Carrie Bearden, a professor in the Departments of Psychiatry and Psychology at UCLA, and Sebastien Jacquemont, a medical geneticist from the University of Montréal, will take advantage of large clinical, neurocognitive and neuroimaging datasets on patients that carry specific gene mutations.
Their teams will collect samples from these patients, which will be converted into stem cells and neurons. This will enable the researchers to correlate neural traits observed in the patient-derived neurons in the laboratory with clinical features of the same patients.

Finally, Sebat will lead the data coordinating center. He and Trey Ideker, a professor in the Departments of Medicine and Bioengineering and Moores Cancer Center Member, will analyze data on genes, pathways and neuronal function.
Using a variety of statistical and machine-learning approaches, they will identify convergent pathways and neurodevelopmental traits between hundreds of genes associated with conditions like schizophrenia and autism. This could help explain how gene mutations influence behaviors and cognitive function, deepening our understanding of these disorders.

By integrating clinical data, genetic analysis and functional studies of neurons and brain organoids, the researchers aim to uncover the biological mechanisms that drive schizophrenia and autism. These discoveries could pave the way for improved diagnosis and treatment of psychiatric disorders in the future.

CIRM, one of the world’s largest institutes dedicated to regenerative medicine, approved $67.5 million in total funding to support five projects through its pilot ReMIND program, a new initiative designed to accelerate the discovery of mechanisms underlying neuropsychiatric disorders leading to the identification and validation of novel targets and biomarkers. The awards correspond to the first, DISC4/ReMIND-L, that has been designed to support large collaborative multidisciplinary projects and integrated studies led by large collaborative teams applying a range of technologies and approaches.
Share This:
You May Also Like
Engineers Take a Closer Look at How a Plant Virus Primes the Immune System to Fight Cancer
Technology & EngineeringStay in the Know
Keep up with all the latest from UC San Diego. Subscribe to the newsletter today.